棉花作为我国重要的经济作物在全国范围内广泛种植,主要有3个种植区,分别为黄河流域棉区,包括山东、河北、河南、天津、陕西、山西、辽宁等省份;长江流域棉区,包括江苏、安徽、湖北、湖南、浙江、四川等省份;西北内陆棉区,包括新疆维吾尔自治区和甘肃等省份。新疆由于气候条件干旱少雨、日照充足,再加上地理优势,具有平坦广袤的土地,逐渐成为我国最大的棉花种植区。据统计,2023年新疆地区的棉花种植面积约为236.93万公顷,占全国棉花总种植面积的84.98%,产量为511.2万吨,约占全国总产量的90.99%[1]。由土传性病原真菌大丽轮枝菌(Verticillium dahliae Kleb)引起的黄萎病是棉花最主要的病害。棉花黄萎病又被称为棉花“癌症”,严重影响棉花的产量和品质。本课题组长期以大丽轮枝菌为研究对象,开展了大丽轮枝菌与植物的互作机制研究。本文结合本实验室的研究和国内外黄萎病最新研究进展,对该病的发生机制和防控技术进行了综述,客观分析黄萎病防治中面临的挑战和亟须解决的主要问题。
1 大丽轮枝菌的生理特征和分类轮枝菌属由广泛的子囊真菌群组成,其中包含了一些能够引起植物维管束萎蔫的病原真菌,最著名的两种就是大丽轮枝菌(V. dahliae)和黑白轮枝菌(V. albo-atrum)[2]。这篇综述主要关注大丽轮枝菌。大丽轮枝菌在分类学上属于子囊菌门(Ascomucota)、粪壳菌纲(Sordariomycetes)、轮枝菌属(Verticillium)[3]。在显微镜下观察该菌可见细长的分生孢子梗,排列成轮枝状;分生孢子位于分枝顶端,形态呈卵圆形。大丽轮枝菌可以产生黑色的微菌核,该休眠体结构可以在土壤中存活数十年之久,一旦遇到宿主就会迅速萌发,形成初侵染源[4]。一直以来大丽轮枝菌被认为是严格的无性生殖,因为在实验条件下从未观察到它的有性生殖结构或减数分裂过程[5]。然而,对于大丽轮枝菌是否具有有性生殖能力一直存在较大的争议,这是因为在大丽轮枝菌中存在2种亲和交配型位点(MAT1-1-1和MAT1-2-1)[6, 7]。子囊菌有性生殖是由多种相关基因决定的,其中最重要的就是MAT基因座的两种类型,编码子囊真菌中有性生殖的重要调节因子[8, 9]。一种类型含有MAT1-1-1基因,另外一种含有MAT1-2-1基因,这两种基因在不同大丽轮枝菌中被发现,因此大丽轮枝菌被认为仍具有有性生殖的能力[10]。但是部分学者认为,虽然大丽轮枝菌存在进行有性生殖的两种配型,但是MAT1-2-1配型所占比例更高,正是由于这种偏好性导致大多数种群进行无性生殖[7, 11]。关于大丽轮枝菌是进行无性生殖还是有性生殖,目前还未得到明确的定论,因此还需要更多更深入的研究来揭示。
2 大丽轮枝菌的侵染过程随着现代生物技术的不断发展,绿色荧光蛋白(GFP)可以通过真菌转化技术在真菌中进行表达,这为真菌的研究提供了非常大的便利。另外,共聚焦显微镜的出现也大大加快了真菌-植物互作研究的进程。通过在真菌中表达GFP蛋白,在共聚焦显微镜下研究者观察到了大丽轮枝菌侵染植物的动态过程。大丽轮枝菌的初侵染源来自微菌核:在温度和pH合适的土壤环境中,受到植物根系分泌物的诱导,存活在土壤中的微菌核迅速萌发,形成菌丝侵入植物根部组织(图 1)。大丽轮枝菌在根部的定植通常发生在根尖或是根尖附近,或沿着根毛到达根表面[12, 13]。真菌在入侵时会在植物接触面形成特殊的入侵结构,例如稻瘟病菌会产生附着胞,凭借其强大的膨胀压力,成熟的附着胞有效地穿透宿主细胞,实现快速入侵[14, 15]。长期以来对于大丽轮枝菌中是否存在相似的侵染结构一直不明确。直到2016年,中国科学院微生物研究所郭惠珊研究员团队的研究表明,大丽轮枝菌同样会产生类似于稻瘟病菌附着胞的侵染结构,其接触到根表面的菌丝会分化出附着枝,进一步发育成侵染钉,完成对根部的入侵。概括来说,大丽轮枝菌的入侵过程包括:附着枝中的VdNOxB/VdPls1基因介导活性氧ROS的爆发,通过提高钙离子的积累激活转录因子VdCrz1的信号转导,促进侵染钉的形成[16]。当大丽轮枝菌入侵到微管组织后,分生孢子会在木质部大量繁殖,并随着蒸腾作用向顶部移动,最终侵染整株植物。在宿主死亡后,大丽轮枝菌产生新的微菌核,潜伏在土壤中,等待新宿主出现后进行下一轮入侵[12, 13]。
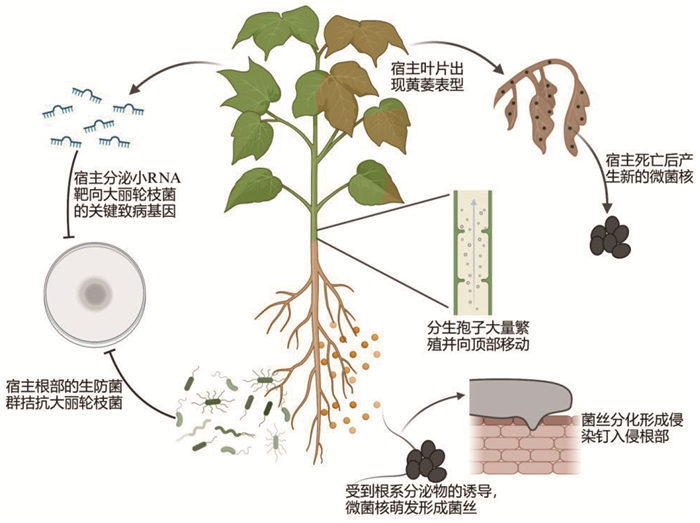
|
图 1 棉花黄萎病的病害循环以及主要防治途径 |
大丽轮枝菌的致病机理十分复杂,对于其致病机理不同的学者持有不同的观点。早期的研究主要集中在两种观点:导管堵塞学说和毒素学说。导管堵塞学说认为,大丽轮枝菌在入侵宿主后,分生孢子在导管内形成菌丝体,从而引起导管堵塞,影响水分和营养物质的运输,导致宿主出现萎蔫。但是部分研究表明,虽然被侵染的宿主导管内出现了菌丝体,但这并不会完全阻断水分和养分的运输[17]。毒素学说认为,大丽轮枝菌会产生毒素,影响宿主代谢,导致植株死亡[18, 19]。但是该毒素具体包含什么物质,目前还没有明确。
最近几年,生物技术的快速发展使我们对大丽轮枝菌的致病机理有了新的认识。基于大丽轮枝菌全基因组测序数据,科研人员开展了大丽轮枝菌致病基因的相关分析。研究发现,VdPKS1参与了大丽轮枝菌黑色素的形成,该基因的缺失不仅会影响微菌核的产生,而且还大大降低了大丽轮枝菌的毒力[20]。效应因子是病原真菌抑制宿主抗性并促进发病机制的毒力因子。目前,在大丽轮枝菌中已鉴定出多种与毒力相关的效应因子。例如:缺少信号肽的VdISc1,可以传递到宿主细胞内,对水杨酸前体进行水解,从而抑制水杨酸代谢[21];富含半胱氨酸的VdSCP7可以被传递到宿主细胞核中,通过未知的机制影响宿主的防御反应[22];分泌蛋白VdSCP41作为胞外效应因子,可以结合并抑制转录因子CBP60的活性,降低宿主植物的免疫响应[23];VdSSP1也参与了对棉花的致病过程,该蛋白具有果胶酶和淀粉酶活性,可以降解植物细胞壁成分,是大丽轮枝菌一个重要的毒力因子[24];此外,本课题组的研究发现,VdSSR1可以转移到植物细胞核内,干扰AGO1-miRNA复合物的出核转运,导致细胞质内AGO1蛋白和miRNA水平大幅下降,从而抑制植物miRNA在大丽轮枝菌内的积累及其诱导的跨界RNAi,增强自身对宿主植物的毒性[25]。表 1对大丽轮枝菌中已鉴定的毒力因子进行了总结。尽管上述研究对大丽轮枝菌的致病基因进行了解析,但是要全面阐明该病原菌致病机理还需要挖掘更多的致病基因,开展更深入的研究。
| 表 1 大丽轮枝菌的一些分泌蛋白 |
棉花黄萎病作为一种土传病害,目前还缺乏相对有效的防治手段。一方面是由于土传病原菌主要是分布在地下,在作物发病时无法像气传病原菌一样大规模喷洒农药,同时由于土壤、根际微生物群落组成十分复杂,并不只包含单一的病原菌,常形成复合侵染,单一的防治手段效果不佳。棉花黄萎病的防治目前主要通过以下几种方式,分别是:化学防治、抗病品种的筛选、农药防治以及生物防治。由于微菌核能够长期在土壤中存活,过去很长一段时间,利用溴甲烷对土壤进行熏蒸成为黄萎病防治不可或缺的手段[50],但是由于种植面积较大,土壤熏蒸的成本较高,同时该方法会在土壤中残留大量的溴甲烷,不利于土地的可持续发展。科研工作者也研发出针对棉花黄萎病的农药,例如克萎星,通过调节发病叶片内AOS代谢,诱导系统性抗性,增加SOS、POD活性,从而减轻膜脂过氧化造成的伤害[51],但是该农药主要对发病前和发病初期有效果,对于发病后期的棉花防治效果较差,主要起到预防的作用。筛选抗黄萎病棉花品种也是我国科研工作者长期开展的工作,但是目前有效的抗病高产自然品种还比较匮乏。值得注意的是,宿主诱导的基因沉默技术(host-induced gene silencing, HIGS)在控制棉花黄萎病方面展现出广阔的潜力。中国科学院微生物研究所郭惠珊研究员团队经过长期的努力,首次证明植物可以针对真菌利用天然小RNA诱导跨界RNAi。该研究发现,感染黄萎病的棉花会诱导微小RNA miR166和miR159的积累,并将其传递到大丽轮枝菌细胞中,靶向沉默关键致病基因Clp-1和HiC-15[52],从而获得跨界RNAi抗性。这一发现为HIGS的利用提供了理论基础。基于此机制,该团队培育出一系列抗黄萎病的棉花株系。该团队还发现,Hydrophobinl (VdH1)疏水蛋白基因作为大丽轮枝菌潜在的致病因子被沉默后,菌株致病力显著下降,以该基因为靶标构建的RNAi转基因棉花同样对黄萎病表现出高效的抗性[53]。此外,本团队首次在植物病原真菌中发现了能够抑制棉花跨界RNAi免疫的效应因子SSR1,该发现为提高跨界RNAi免疫的效率奠定了基础,在育种中可以通过同时靶向致病基因和沉默抑制子的双靶向策略提高HIGS的效率。
生物防治因具有绿色无污染的特点在防治植物病害方面具有独特的优势,在多种植物病害防治中得到应用。生防菌中芽孢杆菌的研究较多,例如有研究表明,芽孢杆菌FJAT-46737、GB03对由细菌引起的番茄青枯病和斑点病具有良好的防治效果[54, 55],解淀粉芽孢杆菌MEP218和ARP23对由核盘菌引起的大豆茎枯病防治效果较好[56]。此外,假单胞菌对由禾谷镰刀菌引起的赤霉病以及由稻瘟病菌引起的稻瘟病也表现出较好的防治效果[57, 58]。尽管在实验室条件下,单一的生防菌株能够有效防治植物病害,但是在自然环境中的防治效果会大大削减,因此复合生防菌群的概念被提出。使用生防菌群不仅能避免单一菌株失效,并且菌群之间也会通过相互作用促进定植。要想利用复合菌群就需要有丰富的生防菌库,而植物根际定植了种类丰富的微生物群落,是很好的生防菌群库[59-61],并且由于大丽轮枝菌是一种土传真菌,根际土壤中可能存在其天然的拮抗微生物群落,因此挖掘开发根际微生物是今后生物防治的研究重点。
4 展望由大丽轮枝菌引起的黄萎病具有极强的破坏性,并且该病原菌宿主范围广,除了可以侵染棉花,还可以侵染番茄、生菜等200多种农作物。该病原菌主要通过破坏植物的维管组织引起植株的萎蔫,因此植物一旦发病几乎不可治愈,只能在前期进行预防。然而该病原菌又能产生微菌核结构,该结构可以在土壤中存活数十年之久,这使得防治变得十分困难。RNAi转基因抗病棉花品种的培育以及利用生防菌进行生物防治是两种比较有效的防治手段,因此接下来需要在大丽轮枝菌关键致病基因上深入开展研究,寻找到更多更有效的RNAi靶标,同时也需要加大生防菌库的挖掘,开发黄萎病生防菌群。此外,从相近物种中寻找有效的抗性基因进行异源表达也是培育抗黄萎病棉花种质的一种可探索的有效途径。
| [1] |
毕梅丽, 刘新. 农小蜂: 2024年中国棉花生产及成本收益分析简报[EB/OL]. (2024-07-04). https://www.abeedata.com/home/article/detail/id/24424
|
| [2] |
Pegg GF, Brady BL. Verticillium wilts: New York: CABI Publishing, 2022: 432.
|
| [3] |
Fradin EF, Thomma BP. Physiology and molecular aspects of Verticillium wilt diseases caused by V dahliae and V. albo-atrum. Mol Plant Pathol, 2006, 7: 71-86. DOI:10.1111/j.1364-3703.2006.00323.x |
| [4] |
Krikun J, Bernier CC. Morphology of microsclerotia of Verticillium dahliaein roots of gramineous plants. Morphology of microsclerotia of Verticillium dahliae in roots of gramineous plants. Can J Plant Pathol, 1990, 12: 439-41. DOI:10.1080/07060669009500988 |
| [5] |
de Jonge R, Bolton MD, Kombrink A, et al. Extensive chromosomal reshuffling drives evolution of virulence in an asexual pathogen. Genome Res, 2013, 23: 1271-82. DOI:10.1101/gr.152660.112 |
| [6] |
Short DP, Gurung S, Hu X, et al. Maintenance of sex-related genes and the co-occurrence of both mating types in Verticillium dahliae. PLoS One, 2014, 9: e112145. DOI:10.1371/journal.pone.0112145 |
| [7] |
Usami T, Itoh M, Amemiya Y. Mating type gene MAT1-2-1 is common among Japanese isolates of Verticillium dahliae. Physiol Mol Plant Pathol, 2008, 73: 133-7. DOI:10.1016/j.pmpp.2009.04.002 |
| [8] |
Metzenberg RL, Glass NL. Mating type and mating strategies in Neurospora. Bioessays, 1990, 12: 53-9. DOI:10.1002/bies.950120202 |
| [9] |
Debuchy R, Turgeon BG. Mating-type structure, evolution, and function in Euascomycetes[M]//Kües U, Fischer R. The Mycota Volume Ⅰ: growth, differentiation and sexuality. Berlin: Springer-Verlag, 2006: 293-323
|
| [10] |
Milgroom MG, Jiménez-Gasco Mdel M, Olivares García C, et al. Recombination between clonal lineages of the asexual fungus Verticillium dahliae detected by genotyping by sequencing. PLoS One, 2014, 9: e106740. DOI:10.1371/journal.pone.0106740 |
| [11] |
Atallah ZK, Maruthachalam K, du Toit L, et al. Population analyses of the vascular plant pathogen Verticillium dahliae detect recombination and transcontinental gene flow. Fungal Genet Biol, 2010, 47: 416-22. DOI:10.1016/j.fgb.2010.02.003 |
| [12] |
Vallad GE, Subbarao KV. Colonization of resistant and susceptible lettuce cultivars by a green fluorescent protein-tagged isolate of Verticillium dahliae. Phytopathology, 2008, 98: 871-85. DOI:10.1094/PHYTO-98-8-0871 |
| [13] |
Zhao P, Zhao YL, Jin Y, et al. Colonization process of Arabidopsis thaliana roots by a green fluorescent protein-tagged isolate of Verticillium dahliae. Protein Cell, 2014, 5: 94-8. DOI:10.1007/s13238-013-0009-9 |
| [14] |
Zhang H, Zheng X, Zhang Z. The Magnaporthe grisea species complex and plant pathogenesis. Mol Plant Pathol, 2016, 17: 796-804. DOI:10.1111/mpp.12342 |
| [15] |
Wang ZY, Soanes DM, Kershaw MJ, et al. Functional analysis of lipid metabolism in Magnaporthe grisea reveals a requirement for peroxisomal fatty acid β-oxidation during appressorium-mediated plant infection. Mol Plant Microbe Interact, 2007, 20: 475-91. DOI:10.1094/MPMI-20-5-0475 |
| [16] |
Zhao YL, Zhou TT, Guo HS. Hyphopodium-specific VdNoxB/VdPls1-dependent ROS-Ca2+ signaling is required for plant infection by Verticillium dahliae. PLoS Pathog, 2016, 12: e1005793. DOI:10.1371/journal.ppat.1005793 |
| [17] |
Taboys PW. Association of tylosis and hyperplasia of the xylem with vascular invasion of the hop by Verticillium albo-atrum. Trans Brit Mycol Soc, 1958, 41: 249-60. DOI:10.1016/S0007-1536(58)80037-6 |
| [18] |
Meyer R, Slater V, Dubery IA. A phytotoxic protein-lipopolysaccharide complex produced by Verticillium dahliae. Phytochemistry, 1994, 35: 1449-53. DOI:10.1016/S0031-9422(00)86872-7 |
| [19] |
Chu ZQ, Jia JW, Zhou XJ, et al. Isolation of glycoproteins from Verticillium dahliae and their phytotoxicity. Plant J, 1999, 9: 972-6. |
| [20] |
Zhang T, Zhang B, Hua C, et al. VdPKS1 is required for melanin formation and virulence in a cotton wilt pathogen Verticillium dahliae. Sci China Life Sci, 2017, 60: 868-79. DOI:10.1007/s11427-017-9075-3 |
| [21] |
Liu T, Song T, Zhang X, et al. Unconventionally secreted effectors of two filamentous pathogens target plant salicylate biosynthesis. Nat Commun, 2014, 5: 4686. DOI:10.1038/ncomms5686 |
| [22] |
Zhang Y, Gao Y, Liang Y, et al. The Verticillium dahliae SnodProt1-like protein VdCP1 contributes to virulence and triggers the plant immune system. Front Plant Sci, 2017, 8: 1880. DOI:10.3389/fpls.2017.01880 |
| [23] |
Qin J, Wang K, Sun L, et al. The plant-specific transcription factors CBP60g and SARD1 are targeted by a Verticillium secretory protein VdSCP41 to modulate immunity. Elife, 2018, 7: e34902. DOI:10.7554/eLife.34902 |
| [24] |
Liu SY, Chen JY, Wang JL, et al. Molecular characterization and functional analysis of a specific secreted protein from highly virulent defoliating Verticillium dahliae. Gene, 2013, 529: 307-16. DOI:10.1016/j.gene.2013.06.089 |
| [25] |
Zhu C, Liu JH, Zhao JH, et al. A fungal effector suppresses the nuclear export of AGO1-miRNA complex to promote infection in plants. Proc Natl Acad Sci U S A, 2022, 119: e2114583119. DOI:10.1073/pnas.2114583119 |
| [26] |
Tzima AK, Paplomatas EJ, Rauyaree P, et al. VdSNF1, the sucrose nonfermenting protein kinase gene of Verticillium dahliae, is required for virulence and expression of genes involved in cell-wall degradation. Mol Plant Microbe Interact, 2011, 24: 129-42. DOI:10.1094/MPMI-09-09-0217 |
| [27] |
Gui YJ, Chen JY, Zhang DD, et al. Verticillium dahliae manipulates plant immunity by glycoside hydrolase 12 proteins in conjunction with carbohydrate-binding module 1. Environ Microbiol, 2017, 19: 1914-32. DOI:10.1111/1462-2920.13695 |
| [28] |
Tian L, Li J, Huang C, et al. Cu/Zn superoxide dismutase (VdSOD1) mediates reactive oxygen species detoxification and modulates virulence in Verticillium dahliae. Mol Plant Pathol, 2021, 22: 1092-108. DOI:10.1111/mpp.13099 |
| [29] |
Gui YJ, Zhang WQ, Zhang DD, et al. A Verticillium dahliae extracellular cutinase modulates plant immune responses. Mol Plant Microbe Interact, 2018, 31: 260-73. DOI:10.1094/MPMI-06-17-0136-R |
| [30] |
Kombrink A, Rovenich H, Shi-Kunne X, et al. Verticillium dahliae LysM effectors differentially contribute to virulence on plant hosts. Mol Plant Pathol, 2017, 18: 596-608. DOI:10.1111/mpp.12520 |
| [31] |
Han LB, Li YB, Wang FX, et al. The cotton apoplastic protein CRR1 stabilizes chitinase 28 to facilitate defense against the fungal pathogen Verticillium dahliae. Plant Cell, 2019, 31: 520-36. DOI:10.1105/tpc.18.00390 |
| [32] |
Gao F, Zhang BS, Zhao JH, et al. Deacetylation of chitin oligomers increases virulence in soil-borne fungal pathogens. Nat Plants, 2019, 5: 1167-76. DOI:10.1038/s41477-019-0527-4 |
| [33] |
Chen JY, Xiao HL, Gui YJ, et al. Characterization of the Verticillium dahliae exoproteome involves in pathogenicity from cotton-containing medium. Front Microbiol, 2016, 7: 1709. |
| [34] |
de Jonge R, van Esse HP, Maruthachalam K, et al. Tomato immune receptor Ve1 recognizes effector of multiple fungal pathogens uncovered by genome and RNA sequencing. Proc Natl Acad Sci U S A, 2012, 109: 5110-5. DOI:10.1073/pnas.1119623109 |
| [35] |
Snelders NC, Rovenich H, Petti GC, et al. Microbiome manipulation by a soil-borne fungal plant pathogen using effector proteins. Nat Plants, 2020, 6: 1365-74. DOI:10.1038/s41477-020-00799-5 |
| [36] |
Snelders NC, Petti GC, van den Berg GCM, et al. An ancient antimicrobial protein co-opted by a fungal plant pathogen for in planta mycobiome manipulation. Proc Natl Acad Sci U S A, 2021, 118: e2110968118. DOI:10.1073/pnas.2110968118 |
| [37] |
Zhang WQ, Gui YJ, Short DPG, et al. Verticillium dahliae transcription factor VdFTF1 regulates the expression of multiple secreted virulence factors and is required for full virulence in cotton. Mol Plant Pathol, 2018, 19: 841-57. DOI:10.1111/mpp.12569 |
| [38] |
Yang Y, Zhang Y, Li B, et al. A Verticillium dahliae pectate lyase induces plant immune responses and contributes to virulence. Front Plant Sci, 2018, 9: 1271. DOI:10.3389/fpls.2018.01271 |
| [39] |
Wang D, Chen JY, Song J, et al. Cytotoxic function of xylanase VdXyn4 in the plant vascular wilt pathogen Verticillium dahliae. Plant Physiol, 2021, 187: 409-29. DOI:10.1093/plphys/kiab274 |
| [40] |
Zhang Y, Gao Y, Wang HL, et al. Verticillium dahliae secretory effector PevD1 induces leaf senescence by promoting ORE1-mediated ethylene biosynthesis. Mol Plant, 2021, 14: 1901-17. DOI:10.1016/j.molp.2021.07.014 |
| [41] |
Yin Z, Wang N, Pi L, et al. Nicotiana benthamiana LRR-RLP NbEIX2 mediates the perception of an EIX-like protein from Verticillium dahliae. J Integr Plant Biol, 2021, 63: 949-60. DOI:10.1111/jipb.13031 |
| [42] |
Ma A, Zhang D, Wang G, et al. Verticillium dahliae effector VDAL protects MYB6 from degradation by interacting with PUB25 and PUB26 E3 ligases to enhance Verticillium wilt resistance. Plant Cell, 2021, 33: 3675-99. DOI:10.1093/plcell/koab221 |
| [43] |
Zhou BJ, Jia PS, Gao F, et al. Molecular characterization and functional analysis of a necrosis- and ethylene-inducing, protein-encoding gene family from Verticillium dahliae. Mol Plant Microbe Interact, 2012, 25: 964-75. DOI:10.1094/MPMI-12-11-0319 |
| [44] |
Wang D, Tian L, Zhang DD, et al. Functional analyses of small secreted cysteine-rich proteins identified candidate effectors in Verticillium dahliae. Mol Plant Pathol, 2020, 21: 667-85. DOI:10.1111/mpp.12921 |
| [45] |
Song Q, Han S, Hu S, et al. The Verticillium dahliae effector VdPHB1 promotes pathogenicity in cotton and interacts with the immune protein GhMC4. Plant Cell Physiol, 2024, 65: 1173-83. DOI:10.1093/pcp/pcae043 |
| [46] |
Qiu P, Zheng B, Yuan H, et al. The elicitor VP2 from Verticillium dahliae triggers defence response in cotton. Plant Biotechnol J, 2024, 22: 497-511. DOI:10.1111/pbi.14201 |
| [47] |
Li Y, Song S, Chen B, et al. Deleting an xylosidase-encoding gene VdxyL3 increases growth and pathogenicity of Verticillium dahlia. Front Microbiol, 2024, 15: 1428780. DOI:10.3389/fmicb.2024.1428780 |
| [48] |
Wang Y, Liao X, Shang W, et al. The secreted feruloyl esterase of Verticillium dahliae modulates host immunity via degradation of GhDFR. Mol Plant Pathol, 2024, 25: e13431. DOI:10.1111/mpp.13431 |
| [49] |
Liu S, Liu R, Lv J, et al. The glycoside hydrolase 28 member VdEPG1 is a virulence factor of Verticillium dahliae and interacts with the jasmonic acid pathway-related gene GhOPR9. Mol Plant Pathol, 2023, 24: 1238-55. DOI:10.1111/mpp.13366 |
| [50] |
Klosterman SJ, Atallah ZK, Vallad GE, et al. Diversity, pathogenicity, and management of Verticillium species. Annu Rev Phytopathol, 2009, 47: 39-62. DOI:10.1146/annurev-phyto-080508-081748 |
| [51] |
胡玉香, 翟长庚, 孙风云, 等. 克萎星对棉花黄萎病的防治效果. 农药科学与管理, 2003, 24: 27-8. |
| [52] |
Zhang T, Zhao YL, Zhao JH, et al. Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nat Plants, 2016, 2: 16153. DOI:10.1038/nplants.2016.153 |
| [53] |
Zhang T, Jin Y, Zhao JH, et al. Host-induced gene silencing of the target gene in fungal cells confers effective resistance to the cotton wilt disease pathogen Verticillium dahliae. Mol Plant, 2016, 9: 939-42. DOI:10.1016/j.molp.2016.02.008 |
| [54] |
Chen M, Wang J, Liu B, et al. Biocontrol of tomato bacterial wilt by the new strain Bacillus velezensis FJAT-46737 and its lipopeptides. BMC Microbiol, 2020, 20: 160. DOI:10.1186/s12866-020-01851-2 |
| [55] |
Alvarez F, Castro M, Príncipe A, et al. The plant-associated Bacillus amyloliquefaciens strains MEP218 and ARP23 capable of producing the cyclic lipopeptides iturin or surfactin and fengycin are effective in biocontrol of sclerotinia stem rot disease. J Appl Microbiol, 2012, 112: 159-74. DOI:10.1111/j.1365-2672.2011.05182.x |
| [56] |
Umesha S. Plant health improvement by Bacillus subtilis strain GBO3 in tomato against bacterial spot disease. J Phytopathol, 2010, 63: 127-30. |
| [57] |
Chen Y, Wang J, Yang N, et al. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat Commun, 2018, 9: 3429. DOI:10.1038/s41467-018-05683-7 |
| [58] |
Yang R, Shi Q, Huang T, et al. The natural pyrazolotriazine pseudoiodinine from Pseudomonas mosselii 923 inhibits plant bacterial and fungal pathogens. Nat Commun, 2023, 14: 734. DOI:10.1038/s41467-023-36433-z |
| [59] |
Venturi V, Keel C. Signaling in the rhizosphere. Trends Plant Sci, 2016, 21: 187-98. DOI:10.1016/j.tplants.2016.01.005 |
| [60] |
Yin C, Casa Vargas JM, Schlatter DC, et al. Rhizosphere community selection reveals bacteria associated with reduced root disease. Microbiome, 2021, 9: 86. DOI:10.1186/s40168-020-00997-5 |
| [61] |
Mendes R, Garbeva P, Raaijmakers JM. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev, 2013, 37: 634-63. DOI:10.1111/1574-6976.12028 |
 2025, Vol. 37
2025, Vol. 37 

 段成国,中国科学院分子植物科学卓越创新中心研究员、博士生导师,国家海外人才引进计划青年项目入选者。2008年获得中国科学院微生物研究所博士学位,2011—2016年在美国普渡大学从事博士后研究,2016年11月起在中国科学院上海生命科学研究院植物逆境生物学研究中心任职。实验室研究兴趣聚焦植物表观遗传机制及其对植物-病原微生物互作的调控,在DNA甲基化、组蛋白修饰、非编码RNA、植物抗病机理及棉花黄萎病致病机制等领域具有丰富的研究经验。近五年来,在Nature Communications、PNAS、Molecular Plant、New Phytologist、Cell Reports等国际核心学术期刊发表 20余篇研究论文
段成国,中国科学院分子植物科学卓越创新中心研究员、博士生导师,国家海外人才引进计划青年项目入选者。2008年获得中国科学院微生物研究所博士学位,2011—2016年在美国普渡大学从事博士后研究,2016年11月起在中国科学院上海生命科学研究院植物逆境生物学研究中心任职。实验室研究兴趣聚焦植物表观遗传机制及其对植物-病原微生物互作的调控,在DNA甲基化、组蛋白修饰、非编码RNA、植物抗病机理及棉花黄萎病致病机制等领域具有丰富的研究经验。近五年来,在Nature Communications、PNAS、Molecular Plant、New Phytologist、Cell Reports等国际核心学术期刊发表 20余篇研究论文