植物在生长过程中受到细菌、真菌、卵菌、病毒等多种病原微生物的威胁,其引发的植物病害会对植物生长和农业生产造成严重威胁。19世纪40年代爱尔兰和欧洲大陆发生的马铃薯晚疫病以及20世纪初美国的板栗疫病等,均对农业和社会经济产生了深远影响。1960年,Russell B. Stevens[1]在总结前人研究的基础上,提出了植物病理学领域著名的“病害三角”理论(disease triangle),即植物病害的发生需要三个必要条件:感病的宿主、有致病力的病原菌,以及适宜的环境条件。这一理论为植物病害的研究提供了重要的框架。在全球气候变暖的背景下,气温升高、降水模式改变、大气中二氧化碳浓度增加以及土壤盐分水平上升等环境变化,不仅显著影响植物的生长和生产力,还以多种方式改变植物与病原菌的互作关系[2, 3]。近年来的研究进一步揭示了不同环境因素调控植物与病原菌互作背后的分子机理。
植物已经进化出两层先天免疫系统来对抗多种病原微生物的入侵。第一层免疫系统是由位于细胞表面跨膜的模式识别受体(pattern recognition receptors, PRRs)通过识别保守的病原相关分子模式(pathogen associated molecular patterns, PAMPs;例如细菌的鞭毛蛋白、真菌的细胞壁几丁质等)而触发的植物免疫,称为模式触发的免疫(pattern-triggered immunity, PTI)。另一层免疫系统是由位于胞内的含有核苷酸结合结构域、富含亮氨酸重复序列的受体蛋白(nucleotide binding, leucine-rich repeat proteins, NLR)直接或间接地识别病原菌的效应因子(effectors)从而引起的植物免疫,称为效应因子触发的免疫(effector triggered immunity, ETI)。PTI和ETI的激活都会引起诸多的下游响应事件,包括钙离子内流、转录重排、活性氧爆发、丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)级联通路以及水杨酸(salicylic acid, SA)、茉莉酸(jasmonic acid, JA)、乙烯等抗病激素的合成等,导致植物抗病性。另一方面,病原菌依赖多种致病因子实现侵染,包括效应子蛋白(一类由不同病原菌产生的毒性蛋白,可由革兰氏阴性细菌Ⅲ型分泌系统或者真菌、卵菌特化结构转运到植物细胞内或者存在于植物细胞外发挥作用)、小分子毒素、细胞壁降解酶等[4]。
现有研究表明,多种环境因素既能够影响植物的免疫响应,也能通过改变病原菌理想生态位的建立、促进效应子或其他致病因子的产生等来影响病原菌致病力,从而对病害产生复杂的调控(图 1)。本综述总结了环境中的非生物因子和生物因子对植物免疫系统和微生物毒力策略的调控,并探讨了未来气候变化中可能导致的非生物胁迫如何重塑对植物病害三角概念的理解。全面理解气候变化如何在机制层面影响植物免疫、微生物致病性及环境之间的相互作用,有助于设计和实施新型应对策略,从而增强植物对气候变化的适应性。
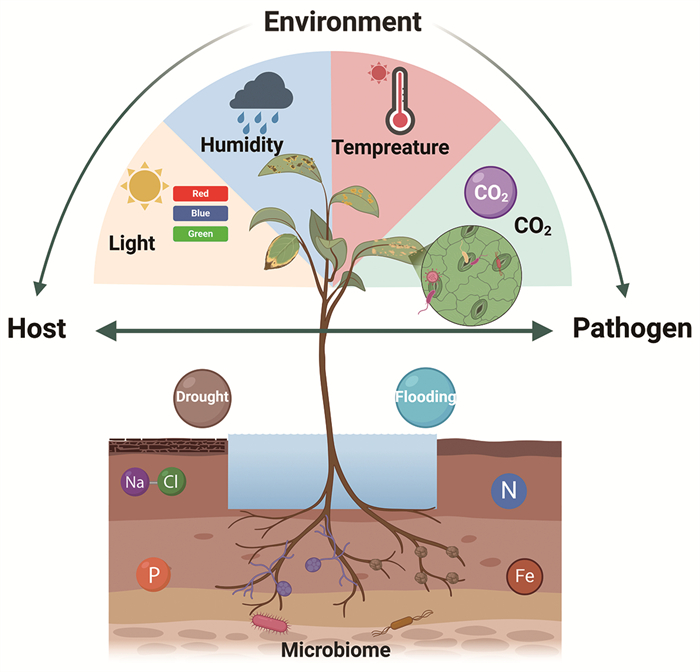
|
植物体受到不同环境条件的影响,地上部分的环境条件从左到右分别是光照、空气湿度、温度以及二氧化碳浓度,地下部分则包括干旱与盐胁迫、水淹、氮/磷/铁营养元素和微生物等。 图 1 环境因素影响植物免疫和病原菌致病力 |
全球变暖导致的温度升高是气候变化的主要非生物胁迫因素之一[5-7]。根据预测,到本世纪末,全球地表平均温度相较于工业化前水平可能升高1.5 ℃至4.8 ℃[8]。气候变化带来的平均气温升高可能会通过增强病原菌的致病性以及抑制植物的免疫响应等增加植物病害的发生[9-11]。
1.1 温度升高对植物免疫的影响由于不同植物物种生长的适宜温度不同,故其对应的高温范围也是不同的。对于模式植物拟南芥(Arabidopsis thaliana)来说,报道中常见的高温处理范围主要分为暖温(warm ambient temperature, 22~27 ℃)、高温(high temperature, 27~30 ℃)以及极端高温(extremely high temperature, 37~42 ℃)[12]。目前,绝大多数的研究结果显示高温会抑制植物对病原菌的免疫响应,从而促进病害[5, 12-14]。
1.1.1 高温对植物PTI和ETI的影响在拟南芥中,较高的温度(28 ℃)能增强由flg22引起的一系列PTI早期信号,如WRKY29和FRK1等PTI标志基因的表达、胞质类受体激酶BIK1 (botrytis-induced kinase 1)的激活和MAPK级联通路磷酸化等,但是会抑制ETI响应[15];反之,较低温度(如16 ℃、23 ℃)下植物的ETI响应增强[15, 16]。然而,不同水平的高温处理对于植物PTI的影响也不尽相同,例如在30 ℃条件下拟南芥对丁香假单胞杆菌Pseudomonas syringae pv. tomato (Pst) DC3000表现出感病的表型,37~42 ℃高温条件也会抑制flg22诱导的PTI响应[17, 18]。
除了调控植物的PTI外,高温也会抑制多种植物中由抗病蛋白介导的ETI免疫。在拟南芥中,高温处理(28 ℃/32 ℃)能显著抑制由多种效应蛋白引起的超敏反应(hypersensitive response, HR)[15, 19, 20]。
目前高温影响植物ETI免疫的分子机制尚不清楚。对烟草中的N和拟南芥中的SNC1 (suppressor of npr1-1, constitutive1)两个NLR的研究显示,高温会减少这两个蛋白的核定位[21]。拟南芥核定位的ZED1 (HopZ-ETI-deficient 1)相关激酶ZRKs (ZED1-related kinases)可通过抑制SNC1的转录来介导温度敏感的免疫反应[22]。温度升高还会诱导转录因子TCPs (teosinte branched 1, cycloidea and proliferating cell factors)如TCP15的表达,TCP15可通过与ZRKs相互作用进一步抑制SNC1的表达[23]。上述结果表明,温度升高可能是通过改变NLRs的核质定位进而抑制ETI。然而,并非所有的NLRs都表现出核定位,甚至有些NLRs的功能在高温条件下是上调的,暗示高温对植物ETI免疫的作用可能存在其他分子机制[24]。
高温还通过NLRs之外的其他基因调控植物ETI免疫。在拟南芥中,植物红光受体光敏色素B (phytochrome B, phyB)作为一个温度传感器,远红光和高温均会抑制其活性[25, 26]。高温会抑制phyB的活性,从而导致光形态建成负调因子DET1/COP1被激活,并促进免疫负调因子PIF4的表达,其能抑制TNL类SNC1的表达[27]。此外,拟南芥的SUMO化修饰(small ubiquitin-like modifier, SUMO) E3连接酶SIZ1在高温下负调控SNC1,同时还会促进SIZ1对COP1的SUMO化修饰,从而增强COP1作为E3泛素连接酶的活性并上调PIF4的蛋白水平,导致高温下ETI免疫被抑制[28]。
除了拟南芥,温度也调控其他作物的抗病性。例如,小麦锈病是全球最具破坏性的病害之一,也是威胁我国小麦安全生产的重要生物灾害,如2017年山东省全省范围内均发生了小麦条锈病,发病面积高达213万公顷,占全部小麦种植面积的54.69%,全省病田率为41.38%,导致小麦减产甚至绝收[29]。温度在小麦条锈病的发生中起着关键作用。小麦条锈菌具有喜低温的习性,易受环境因素的影响。小麦条锈菌适宜的温度范围为0~32 ℃,在高于36 ℃时则失去活力。冬季温暖有利于病菌越冬,返青拔节期遇高温多雨天气则有利于条锈病春季流行[30-32]。富半胱氨酸的类受体激酶(cysteine-rich receptor-like kinases, CRKs)是最大的类受体激酶(receptor-like kinases, RLKs)亚家族之一,参与植物PTI免疫响应。近年来,Wang等[33]从小麦栽培品种“XY 6”中鉴定到了一个能在较高温度(20 ℃,相比于正常温度16 ℃)条件下赋予苗期植物条锈病抗性的基因TaCRK10。研究发现,小麦在高温下感染真菌Puccinia striiformis f. sp. tritici后,体内的TaCRK10表达会被显著诱导,导致组蛋白H2A (TaH2A.1)磷酸化并激活水杨酸信号通路,从而增强高温下苗期小麦对条锈病的抗性。而在番茄中,35 ℃高温处理3 h后,番茄Mi-1基因介导的线虫抗性被显著抑制[34]。此外,烟草N基因和辣椒TSW基因介导的病毒抗性在30 ℃或更高温度下也显著减弱[35]。然而研究显示,高温下植物ETI免疫被抑制似乎也不是普遍规律,例如在小麦中发现NLR基因Sr13和Sr21能赋予植物在高温条件下对小麦秆锈病病原菌(Puccinia graminis f. sp. tritici Ug99)的抗性[36, 37]。此外,某些NLR,如在烟草中表达的马铃薯Rx1基因在30 ℃或更高温度下仍保持活性,植株仍能产生明显的HR,并赋予植物对马铃薯病毒X的抗性[38]。综上所述,温度可通过影响植物的PTI和ETI途径进而调节其抗病性。
1.1.2 高温对植物免疫相关激素的影响研究显示,高温(28~30 ℃)可以抑制由病原菌诱导的SA合成和信号转导[39],从而导致拟南芥以及烟草在高温下分别对丁香假单胞杆菌和烟草花叶病毒(tobacco mosaic virus, TMV)感病[17, 20, 40]。近年一项研究表明,高温(28~30 ℃)条件下拟南芥中SA的合成被抑制主要是由于SA合成途径中的两个关键转录因子基因CBP60g (calmodulin-binding protein 60-like g)和SARD1 (systemic acquired resistance deficient 1)的转录在高温下被抑制[40]。这一过程与热敏的类鸟苷酸结合蛋白(guanylate-binding protein-like, GBPL)防御激活的凝聚体(Gbpl defence-activated condensates, GDACs)的作用有关。高温会抑制GDACs的形成,进而通过降低CBP60g和SARD1的表达抑制SA的合成[40, 41]。此外,研究显示,在拟南芥中过表达NDR1也能通过缓解受高温抑制的RPS2和SA相关基因(包括ICS1和CBP60g)的表达,从而回补植物在高温(29 ℃)条件下的ETI抗性与SA水平[42]。
在单子叶植物水稻中,由细菌稻黄单胞杆菌致病变种(Xanthomonas oryzae pv. oryzicola, Xoc)引起的细菌性条斑病是我国重要的植物检疫性病害之一。根据2023年11月1日农业农村部办公厅印发的《全国农业植物检疫性有害生物分布行政区名录》,水稻细菌性条斑病已传播至全国14个省421个县(市、区),且以华东、华南、华中及西南稻区受影响较为严重,成为我国水稻第四大病害。水稻细菌性条斑病的爆发与气象因素密切相关,高温、高湿及台风、暴雨等有利于水稻细菌性条斑病的爆发与流行。据统计,Xoc侵染水稻后通常导致产量减少10%~20%,发病严重时减产高达40%以上,甚至造成水稻绝收,对我国粮食安全构成严重威胁[43-45]。研究发现,与正常温度(日/夜=29/23 ℃)相比,在高温条件下(日/夜=35/29 ℃)由Xa7基因介导的对细菌条斑病抗性显著增强,但是RNA-seq数据显示在抗病过程中发挥重要作用的植物激素SA信号途径中基因的表达却被高温显著抑制;进一步的研究发现,ABA可能在水稻应对高温胁迫和病原菌侵染所引起的转录响应过程中发挥重要作用[46]。
除了细菌性病害,由病原真菌稻瘟病菌(Magnaporthe oryzae)引起的稻瘟病也是水稻主要病害之一,在我国各水稻栽培区经常发生,年均受害面积达400万公顷以上,导致的产量损失在20亿千克以上,是我国粮食安全生产的重要威胁之一[43, 47]。研究发现,与暖温(22 ℃)相比,正常生长温度(28 ℃)会导致水稻对稻瘟病菌(M. oryzae)的抗性增强:在28 ℃条件下,稻瘟病菌可有效诱导水稻JA生物合成和信号转导,且JA通路通过OsMYB22及其互作蛋白OsMYC2上调水稻基础抗病基因OsCEBiP的表达,进而介导较高温度下的稻瘟菌抗性[48]。而小麦中TaRPM1和TaRPS2等CNLs也是通过SA信号通路正向调节高温下小麦对三尖杉条斑菌(P. striiformis f. sp. tritici)的抗病性[49, 50]。
综上可知,高温对于植物免疫响应的调控在不同植物中有所不同,故在双子叶植物(如拟南芥)研究中发现的分子机制可能并不完全适用于单子叶植物。而在水稻和小麦等作物中,目前很多研究只是从生理层面上描述抗病表型,具体的分子调控机制还需要进一步研究。
1.1.3 高温对病原菌致病力的影响温度可以显著影响病原菌的入侵和定殖效果。例如,高温会诱导某些细菌病原菌(如果胶杆菌)产生更高水平的细胞壁降解酶和群体感应信号来增强毒力[51]。此外,高温能通过改变病原菌分泌系统活性,从而影响其致病性,如农杆菌的Ⅳ型分泌系统在32 ℃或更高的温度下会失活[52, 53]。对于革兰氏阴性细菌的Ⅲ型分泌系统(T3SS)而言,有研究表明低温(18~22 ℃)可在体外上调T3SS相关基因的表达[54],而高温(30 ℃)处理则增强Pst DC3000效应因子向植物细胞内的转运[17, 55]。除细菌外,温度可作用于丝状病原真菌的不同生长阶段[56, 57]。温度和湿度对稻瘟菌孢子的形成、萌发和侵染起到重要作用。当气温在24~28 ℃、相对湿度在90%以上时,分生孢子发芽侵入的时间最短,只需6~8 h,而潜育期为4 d;当气温在17~18 ℃时,潜育期8 d;当气温超过30 ℃时,则不利于病菌生长,病害停止发展[58]。此外,一个保守的温度调控分子伴侣HSP90对禾谷镰刀菌无性和有性孢子的产生至关重要[59]。高温(超过30 ℃)会抑制丝状病原真菌附着孢子体的形成,从而影响侵染过程[60]。此外,假禾谷镰刀菌(Fusarium pseudograminearum)作为引起小麦茎基腐病的坏死性植物病原菌,其在15/15 ℃条件下表现出最佳的总体适应性,随着昼夜温差的增大(20/15 ℃、25/15 ℃或28/15℃),假禾谷镰刀菌的适应性逐渐降低,表明环境温度的变化会影响病原菌的适应性与致病力[61]。
2 空气湿度空气湿度是生产实践中对植物病害具有深远影响的环境因素,高空气湿度条件(相对空气湿度,relative humidity,RH > 90%)能促进一系列植物病害的爆发与流行。在自然界(热带亚热带地区)以及温室大棚、组织培养等农业生产系统中,高空气湿度都是常见的环境条件[62-64]。已有研究指出,高空气湿度能够通过抑制植物的免疫响应或增强病原菌的致病力等,促进植物地上部分由细菌、真菌或卵菌引起的各种病害[65-67]。
2.1 高空气湿度影响植物的免疫响应 2.1.1 高湿度对植物免疫相关激素的影响高湿度条件能改变植物的多种生理状态和生物学过程,其中包括植物激素。在自然界中,病原菌进入植物地上部组织内部主要是通过自然的开孔,如植物的气孔(stomatal)以及水孔(hydathodes)。在植物的PTI响应过程中,叶片上的气孔会关闭以阻止病原菌的进一步侵染[68, 69],这一过程依赖于SA。已有报道显示,高湿度条件能通过上调拟南芥以及豆类植物保卫细胞中的JA信号并下调SA信号从而抑制植物的气孔免疫[66]。在拟南芥中,高湿度通过抑制植物的泛素化途径降低SA的关键受体NPR1的泛素化水平以及NPR1作为转录共激活子结合靶基因启动子的能力,从而抑制SA的信号途径,促进感病性[67]。在水稻与稻瘟病菌的病害模型中,与正常湿度(70% RH)相比,高湿降低乙烯前体物1-氨基环丙烷-1-羧酸(ACC)的积累和乙烯响应基因的表达,进而增强稻瘟菌的侵染[70]。而在拟南芥中,仅高湿处理(无病原菌侵染)就可以诱导乙烯的积累与信号转导[71]。
2.1.2 高空气湿度影响植物的超敏反应早期的报道指出,高湿处理会显著抑制由R蛋白如Cf-4或Cf-9在番茄中引起的超敏反应或者拟南芥中自免疫突变体产生的细胞死亡[72-74]。此外,在拟南芥自免疫突变体ssi4中,高湿还能抑制活性氧ROS积累、MAPK级联通路等其他自免疫表型[73]。虽然高湿能抑制植物的HR,但从细菌的生长上看,高湿条件下植物的ETI抗病性仍然存在,因此高空气湿度调控植物ETI通路的机制仍有待进一步研究。
2.2 高空气湿度影响病原菌致病力高空气湿度除了能影响植物的免疫,还能通过增强病原菌的致病力来促进植物病害的发生。在温差大的地区或者梅雨季节,空气湿度局部升高会导致作物冠层形成露水,使得病原菌的鞭毛蛋白运动性增强,从而促进叶际附生病原菌通过气孔向宿主内部定殖和侵染[75]。此外,在高湿度环境中,许多叶片内生病原菌如假单胞菌属和黄单胞菌属会在植物叶片的胞间质(或者质外体,apoplast)中创造液态水环境,即宏观视角下的水渍状病斑,极大促进病原菌的繁殖[76-79]。此外,高湿度也会诱导丁香假单胞菌的操纵子hrpL以及效应因子基因hopD1和hopN1表达水平升高[80]。
高湿度对于许多病原真菌的孢子萌发十分重要。如引起温湿气候型重大流行性病害小麦赤霉病的禾谷镰孢菌(Fusarium graminearum)仅在RH > 75%时产生子囊壳,并仅在RH > 85%时成熟,而且子囊壳的产生和成熟随着RH的增加而增加[81]。因此,在菌量充足的条件下,小麦抽穗扬花期如遇2 d以上的连阴雨天气,病害就可能爆发成灾。历史上长江中下游、江淮麦区为小麦赤霉病的常发区,常年发生面积267~333万hm2。赤霉病一般流行年份可以引起10%~20%的产量损失,大流行年份可导致绝收,同时病菌产生呕吐毒素、玉米赤霉烯酮等多种真菌毒素污染麦粒,导致小麦质量下降,甚至失去食用或饲用价值[47, 82, 83]。除此之外,稻瘟菌等病原真菌的孢子萌发也依赖于高湿,因此高空气湿度也是导致许多真菌病害爆发的主要环境因素之一[84-86]。
3 光照光是植物光合作用的能量来源和调控植物生长发育的重要信号。植物进化出一系列光受体感知不同波长的光信号,如感知红光/远红光比例的受体光敏色素(phytochromes, Phys)、感知蓝光的受体隐花素(cryptochromes, CRYs)和向光素(phototropins, PHOTs)等。近年研究发现,光对调控植物免疫和病害有重要作用。如光强减弱(如庇荫条件及增加植物冠层密度)可导致植物对不同病原菌的抗性变弱[87-89])。在拟南芥中,减少红光/远红光比例会显著降低由水杨酸SA及茉莉素JA介导的病原菌抗性,与此相符的是,拟南芥phyA/phyB突变体表现出对SA不敏感的表型,并且其介导的系统获得性抗性显著减弱[90-92]。在番茄中,红光处理同样能够通过增强SA信号提高其对丁香假单胞菌的抗性[93]。研究还发现,在水稻中,phyB介导的光信号通过抑制BZR1-NAC028-CAD8B信号途径降低水稻对纹枯病的抗性;而通过抑制转录因子GT1和PIL15以及下游信号转导来促进水稻对稻瘟病抗性,表明phyB信号对植物应对不同病原菌抗性具有多效性[94, 95]。此外,蓝光信号也调控植物与病原菌互作。前期研究表明,蓝光受体可能参与调控ETI过程中SA响应基因PR1的表达从而介导植物的抗病性[96]。最近一项研究显示,在蓝光条件下,拟南芥蓝光受体CRY1在介导植物气孔打开以进行光合作用的同时,也会上调植物的一个磷脂翻转酶LURP1:LURP1在PAMP处理后增强质膜定位并促进FLS2/BAK1免疫受体复合体的形成和激活,从而增强PTI免疫[97]。另有研究表明,蓝光受体向光素PHOT1负调控INF1介导的免疫响应,从而促进疫霉菌对马铃薯的侵染[98, 99],表明蓝光信号对不同的病害体系可能发挥不同的作用。
此外,丁香假单胞杆菌等病原菌能够分泌效应因子,通过诱导气孔关闭等,使植物质外体形成一种利于病原菌生长的富水环境;而研究发现,持续光照处理会通过上调拟南芥SA信号,抑制丁香假单胞菌诱导的气孔关闭,进而减少质外体中水分的积累,提高抗病性[100]。
图 2总结了气候条件对植物免疫和病原菌致病力的影响。
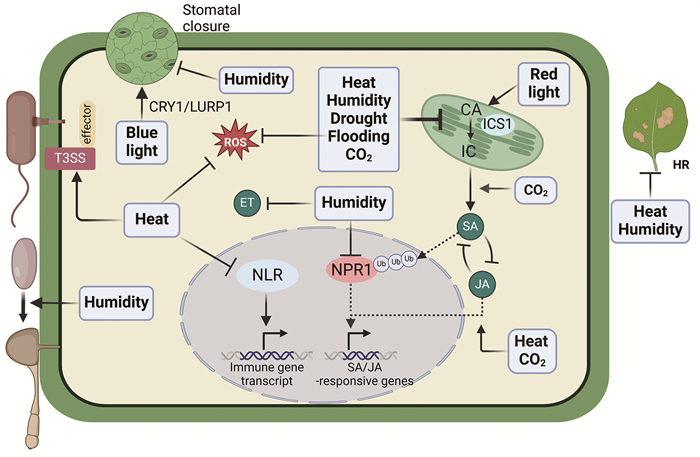
|
高温(heat)能通过影响NLR活性、抑制SA的合成等途径抑制植物的免疫抗性,同时高温还能促进病原菌的效应蛋白分泌,从而增强病原菌的致病力。高空气湿度(humidity)能通过抑制植物的气孔免疫、SA的信号转导、水稻乙烯途径等抑制植物免疫,并且高湿度能促进真菌孢子萌发,从而促进真菌病害的发生。干旱(drought)、水淹(flooding)以及高浓度二氧化碳(CO2)都能通过影响植物的SA水平从而抑制植物的免疫响应。此外,水淹还能抑制拟南芥活性氧的产生,高浓度的CO2能提高JA的水平。拟南芥蓝光受体CRY1能通过促进LURP1蛋白的积累从而增强蓝光条件下植物的PTI免疫,而红光则能促进拟南芥SA的积累,从而增强其抗性。HR,超敏反应;NLR,核苷酸结合富含亮氨酸重复序列;T3SS,Ⅲ型分泌系统;CA,分支酸;IC,异分支酸;ICS1,分支酸合成酶1。 图 2 气候条件对植物免疫和病原菌致病力的影响 |
氮(N)是植物生长和维持基本细胞功能的必需元素,植物主要通过吸收土壤中的无机态氮(硝酸盐NO₃⁻与铵盐NH₄⁺)来合成重要的有机化合物。施用氮肥是农业生产中提高作物产量的关键措施之一,然而过量施用氮肥可造成生态环境破坏,并对作物本身造成负面影响。例如,过量施加氮肥会导致水稻和小麦易感稻瘟病[101, 102],且减氮20%施肥能够降低盐梗系列水稻品种稻瘟病的发生,并且对水稻的产量及品质没有显著的影响[103]。除稻瘟病外,过量施加氮肥也会增加小麦白粉病、条锈病[104]、莴苣灰霉病[105]等生产中常见病害的易感性。但是也有部分研究显示,高浓度的N水平能降低植物对病原菌的易感性,例如高浓度N可以降低番茄灰霉病的病害程度[106, 107],因此N对植物与病原菌的互作的影响取决于具体的互作系统。在农业生产过程中,应及时评估作物的营养生长状态合理施用氮肥,以期实现品质产量的最高化与病害发生的最低化。
在N营养丰富的条件下,植物生长会得到促进,而木质素(可用于强化细胞壁)的合成会受到抑制,从而导致植物的物理防御能力降低,更易感病[108-110]。近年来, 越来越多的研究表明,环境中的N水平与植物免疫防御反应之间存在显著相关性。例如,在低N条件下,拟南芥与Pst DC3000侵染的番茄中会积累更多的水杨酸[111, 112]。硝酸盐转运蛋白(nitrate transporter, NRT)基因可能介导氮素对植物免疫防御反应的调控。例如,与野生型相比,拟南芥nrt2.1/2.2缺失突变体对Pst DC3000的抗性增强,可能是由于该突变体的SA响应增强而对细菌冠毒素(coronatine)的响应减弱[113];而拟南芥nrt2.6突变体对病原细菌梨火疫病菌(Erwinia amylovora)的抗性降低[114]。拟南芥硝酸根还原酶(nitrate reductase)双突变体nia1/2表现出丁香假单胞杆菌诱导的NO (nitric oxide)含量降低且HR减弱的表型[115, 116]。另外,植物会在根部缺N的情况下诱导CEPs (C-terminally encoded peptides)的产生并转运其至地上部以传递N缺乏信号,同时诱导根部NRT基因表达[117, 118]。Rzemieniewski等[119]发现,CEPs与其受体CEPR1/2及RLK7共同参与调节拟南芥中的基础免疫;在低N条件下,flg22诱导的拟南芥PTI响应增强,而这一增强作用依赖于CEPs及其受体,表明CEPs可能是联系N与植物免疫系统的一个重要纽带。此外,有报道显示,拟南芥植物在低N条件下生长时,侵染的E. amylovora病原菌中Ⅲ分泌系统hrp基因上调导致致病性增强[120, 121]。
4.2 磷与植物免疫磷(P)也是植物生长和发育所必需的营养元素之一,植物主要吸收土壤中的无机磷酸盐(Pi)用于自身的生长发育。尽管土壤中的P含量较高,但是可供植物利用的Pi在土壤中的浓度通常较低[122],为了适应Pi缺乏环境,植物进化出了适应性的Pi饥饿反应(phosphate starvation responses, PSRs),包括侧根的快速伸长与Pi饥饿相关转运蛋白及转录调控因子的激活等[123-125]。现代农业生产也广泛施用磷肥来保证作物的高产,但是过度施用磷肥会造成水污染等严重的环境问题。与N类似,环境中Pi的水平也会影响植物与病原菌的互作[126]。例如土壤过量Pi处理会降低水稻的免疫防御反应且促进水稻易感稻瘟病[127];而低浓度Pi会降低拟南芥对Pst DC3000与灰霉菌Botrytis cinerea的抵抗力,但是提升棉花中JA的积累并提升棉花对Verticillium dahliae的抵抗力[128, 129]。
目前对Pi调控植物免疫的机制研究还较少。Khan等[130]发现,拟南芥、番茄与烟草会在低Pi条件下积累更多的JA;而Jaskolowski等[128]发现,低Pi条件下生长的拟南芥或者低Pi突变体pho1表现出的灰霉菌感病性增强,这一过程依赖ABA水平升高以及灰霉菌孢子萌发率上升。这些结果说明,Pi可以通过影响植物激素的合成直接或间接影响植物的抗病性。除此之外,研究者们还发现,PSRs中的关键因子也参与了植物免疫防御反应。与此同时,在拟南芥中,PSRs主要由PHR1 (phosphate starvation response 1)及其同源PHL1 (PHR1 like 1)转录因子所调控。拟南芥的phr1 phhl1双突变体表现出比野生型更强的对Hyaloperonospora arabidopsidis isolate Noco2和Pst DC3000的抗性[127, 131]。研究表明,在Pi饥饿条件下,PHR1直接与RALF (rapid alkalinization factor)基因的启动子结合并上调其表达,RALF多肽通过其受体FERONIA干扰PRR受体复合物的组装,进而抑制PTI免疫[132]。此外,He等[133]发现拟南芥PHR1蛋白能与JA途径核心转录因子MYC2/3/4互作形成复合体,并与多个JA响应基因的启动子结合,从而上调低Pi条件下的JA通路。这些结果都揭示了PHR1在协调外源Pi水平与植物免疫活动中的重要作用。有趣的是,在Pi充足条件下,来自根际细菌的MAMPs,如flg22,可激活拟南芥PRR受体FLS2/BAK1的复合物以及下游的胞质类受体激酶BIK1和PBL1 (PBS-like 1),BIK1/PBL1磷酸化表皮定位的磷酸盐转运蛋白PHT1.4并降低其转运Pi的活性,从而抑制Pi吸收,且拟南芥pht1.4突变体表现出低Pi条件下更抗病的表型[134]。在水稻中,PAMP处理以及稻瘟菌、白叶枯菌侵染会降低水稻高亲和性Pi转运蛋白OsPT8 (phosphate transporter 8)的转录水平,且OsPT8负调控水稻的PTI响应以及稻瘟病、白叶枯病抗性[131]。
此外,有研究报道,高Pi条件也可以加速病原菌的生长和繁殖,提高其侵染能力。例如,稻瘟病菌在高磷条件下,其效应因子BAS4和PWL2的表达显著升高,增强了其在叶片和茎中的致病力[127, 135]。
4.3 铁 4.3.1 铁稳态对植物免疫的影响铁(Fe)作为一种必需的营养元素,对于植物病原菌的生存和致病性至关重要。植物体内的铁含量会显著影响植物的免疫响应。局部的铁积累也是植物的一种铁免疫策略,通过芬顿反应在感染部位募集铁会引发ROS的爆发。例如在小麦-大麦与白粉病(Blumeria graminis)的互作过程中,Fe³⁺会在细胞壁附着点处积累并引起ROS爆发[136]。在水稻与稻瘟病互作的过程中,Fe³⁺与ROS也会在稻瘟病菌的侵染位点积累[137]。在水稻中高浓度外源铁处理还可通过强烈诱导防御相关基因和参与二萜类植物抗毒素和樱花素代谢产物合成相关基因的表达来增强水稻对稻瘟病的抗性[138]。在玉米中,充足铁条件通过增强ROS产生等抑制禾本科炭疽刺盤孢菌(Colletotrichum graminicola)的侵染[139]。此外,有研究报道铁积累可能还会参与由ETI引起的HR过程,这与动物细胞中报道的铁死亡概念(一种细胞死亡途径,依赖于铁和ROS导致的脂质过氧化物在细胞质或质膜中的致死性积累)是相似的。研究发现,水稻中由稻瘟病引起的HR过程总是伴随着显著的ROS积累和铁离子显著富集,并且通过使用铁的螯合剂去铁胺(deferoxamine, DFO)或疏水性抗氧化剂Fer-1 (ferrostatin-1)可以显著减少铁和ROS的积累,包括脂质过氧化产生的丙二醛(MDA),进而抑制ETI过程中的HR反应[137]。
另一方面,也有研究报道铁缺乏能够赋予植物抗病性。例如铁的螯合剂去铁胺能触发拟南芥植物典型的PTI反应,如ROS爆发、胼胝质积累,表明植物免疫可能通过减少铁含量来抵御病原菌侵染[140]。在低铁条件下,Platre等[141]发现低铁条件和flg22处理会诱导一些类似的基因转录变化,并且flg22处理会导致快速的拟南芥根部铁水平下调,这一过程依赖于一个植物受体激酶SRF3 (strubbelig-receptor family 3)。最近研究表明,在缺铁条件下,拟南芥flg22通过铁稳态调节因子BTSL1和BTSL2促进一个铁缺乏信号诱导的小肽——IMA1 (iron man 1)的泛素化和降解,从而抑制植物对铁缺乏的响应和调控铁稳态[142]。这种调控策略不仅限制了根际细菌的铁利用,同时也为植物在免疫响应和养分吸收之间提供了平衡机制。
宿主植物中的许多铁稳态基因也会受到病原菌侵染的调控,并参与植物免疫。研究发现,Erwinia chrysanthemi感染拟南芥叶片后,参与铁信号通路的IRT1、FRO2和AtNRAMP3基因的表达在根部上调,进而增加了拟南芥根中的铁含量[143]。因此,病原菌可能会在宿主植物中激活一种从地上部到根的信号(类似铁缺乏的信号),调节植物的铁吸收与免疫反应[140, 143]。此外,AtFer1 (铁蛋白)的表达在E. chrysanthemi感染拟南芥后也被上调,其上调部分依赖于病原菌铁载体的合成,并且增强植物的免疫[143, 144]。此外,无毒的稻瘟病菌INA168侵染水稻会诱导水稻铁蛋白基因OsFER2的表达,OsFER2的突变体则显示出更少的HR和更严重的感病表型,暗示OsFER2参与了无毒性稻瘟病菌感染过程中铁和ROS依赖的铁死亡过程[145]。上述研究表明,这些铁稳态基因通过调节宿主植物的铁稳态增强植物的抗病能力。
4.3.2 铁对病原菌的影响研究发现,许多病原菌的铁吸收对其致病性有重要作用,病原菌可以利用多种铁吸收途径,包括铁载体介导的铁吸收途径(高亲和型以及低亲和型)和内源铁载体(如RIA途径)介导的铁储存[146]。然而,病原菌操纵植物宿主以获取铁的机制尚不清楚。研究发现,由丁香假单胞菌分泌的效应蛋白AvrRps4与拟南芥中的植物铁感应蛋白BTS (Brutus)相互作用,稳定铁调控因子bHLH115和ILR3 (IAA-leucine resistant 3)的蛋白水平,从而促进铁在叶片质外体空间的积累和病原菌增殖[147]。
图 3总结了氮、磷与铁营养影响植物免疫的分子机制。
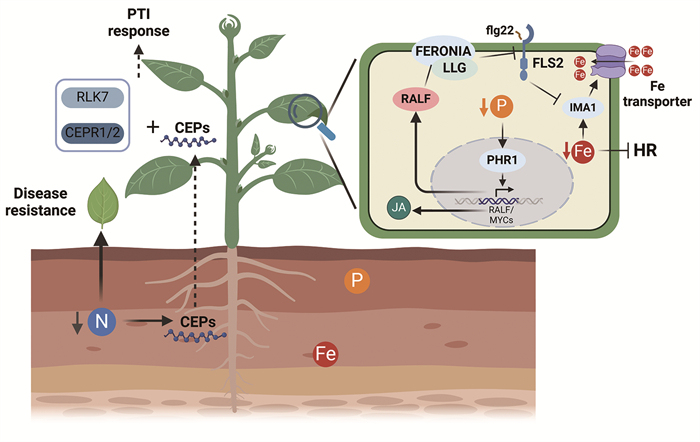
|
土壤氮水平会影响植物免疫。低氮条件会诱导植物根部CEPs小肽合成并运输至地上部,CEPs与其受体CEPR1/2及RLK7共同促进植物的PTI响应。此外,在低氮条件下,植物的SA水平也会升高,使得植物抗性增强。低磷条件会诱导磷饥饿反应(PSRs),其关键转录因子PHR1能通过MYCs蛋白上调JA通路。PHR1还能促进RALF小肽表达,RALF通过其受体FERONIA干扰PRR受体复合体的形成,从而抑制PTI免疫。铁的水平与再分配也会影响植物的免疫。在低铁条件下,植物通过IMA1小肽增强铁摄取,而flg22处理会促进IMA的降解、抑制铁的吸收,铁饥饿还可能会抑制植物的超敏反应。 图 3 氮、磷与铁营养影响植物免疫的分子机制 |
植物在被水淹的状态下由于气体交换困难,会产生缺氧胁迫。在拟南芥中,早期的研究发现在水淹胁迫下,植物能通过转录因子WRKY22来增强自身的免疫响应,从而减少病原菌的侵染[148]。然而,最近研究发现,缺氧对于植物免疫更多的是抑制作用,如在缺氧条件下处理flg22可导致拟南芥PTI转录响应被显著抑制[149]。此外,在马铃薯块茎以及桉树中,缺氧条件下植物防御相关的伸展蛋白(extensin)以及SA合成相关的PAL基因家族的转录被抑制[149-151]。在ETI免疫方面,接种能引起ETI的疫霉菌后,大豆以及烟草在缺氧条件下的ROS爆发以及HR均被抑制[152]。上述结果显示,水淹或许不能简单地等同于缺氧条件,它们对于植物免疫的影响仍有待于更深入的研究。
5.2 水淹和缺氧对病原菌的影响在水淹过程中,植物与病原菌都会经历缺氧。对于部分病原菌来说,缺氧过程可以增强病原菌毒性因子的活性,如果胶杆菌属(Pectobacterium spp.)、迪克氏菌属(Dickeya spp.)和胡萝卜软腐欧文氏菌(Erwinia carotovora)的果胶酸裂合酶在缺氧条件下表现出更高的表达水平与活性[150, 153]。这些病原菌在农业生产中,尤其是在马铃薯和其他根茎类作物中引起了严重的病害。Pectobacterium属的病原菌是马铃薯软腐病的主要致病因子,在缺氧环境下,病原菌活性增加,加剧了病害的扩展,导致大规模的马铃薯腐烂,严重损害产量和品质[154]。此外,迪克氏菌属溶杆状菌(Dickeya solani)在缺氧条件下,其毒性基因表达也会上调,这些结果表明外部缺氧可增强这些病原菌的致病能力[150, 155]。此外,真菌在定殖过程中消耗氧气可能进一步降低宿主氧气水平,从而加剧植物的内部缺氧[156, 157]。例如,在稻瘟病菌侵染水稻时,缺氧诱导的基因表达对稻瘟病菌的致病性具有重要贡献。不同证据表明,相对于植物宿主来说,病原菌可能更适应缺氧环境[158, 159],然而对于水淹胁迫具体是通过何种方式调控病原菌的致病力仍有待进一步的研究。
6 干旱与盐胁迫 6.1 干旱与盐胁迫对于植物免疫的影响在全球变暖的情况下,极端天气的高发使得干旱的发生也更加普遍,长时间的干旱会导致土壤中水分减少、盐浓度升高,并最终导致土壤盐碱化。在大多数情况下,干旱或盐胁迫会促进植物病原菌如水稻白叶枯病菌(Xanthomonas oryzae)、马铃薯链霉菌(Streptomyces spp.)、拟南芥丁香假单胞杆菌的侵染[160-162]。
植物在经历干旱或盐胁迫时会诱导ABA的积累,以应对这一胁迫过程。在大多数情况下,ABA与SA途径是拮抗的[100, 163],从而导致植物在干旱胁迫下免疫响应被抑制[162, 164]。例如在拟南芥中,在接种病原菌Pst DC3000后,干旱处理导致SA的合成以及响应都被抑制,并表现出感病的表型[164]。
此外,研究发现,在干旱胁迫处理后的恢复阶段,植物的免疫响应也同样会产生变化,称为干旱恢复诱导免疫(drought recovery-induced immunity, DRII)。相关报道显示,拟南芥在激活了DRII后会增强其对丁香假单胞杆菌的抗病性;在园艺作物鳄梨中也有报道显示,轻度的干旱处理能增强其对白绢根腐病菌(Rosellinia necatrix)的抗性[165, 166]。这些研究表明,干旱或盐胁迫对于植物免疫响应的作用似乎并不是简单的抑制,两者之间有着更为复杂的互作分子机制。
7 二氧化碳(CO2)胁迫随着化石燃料的使用,大气中的CO2浓度在近代以来不断升高。不同的植物种类、生态型对于CO2浓度升高的响应有着很大的差异性。在番茄中,高浓度的CO2能促进SA的合成及信号转导并抑制JA通路,使得植物对活体营养型病原菌如丁香假单胞杆菌以及烟草花叶病毒TMV表现出更抗病的表型,而对死体营养型病原菌如葡萄灰霉菌表现出叶片黄化坏死程度加剧、光合作用减弱的感病表型[167, 168]。
在拟南芥中,在不同CO2浓度下的免疫响应表现出相反的趋势。800 ppm的CO2浓度会诱导拟南芥叶片ABA的积累促进气孔关闭,并使得植物对丁香假单胞菌更感病,这或许是ABA与SA之间的拮抗所导致的[169]。而另一项研究显示,在3 000 ppm的极高浓度下,拟南芥的SA积累更高,对丁香假单胞菌表现出更抗病的趋势,且植物的鲜重也增加[170]。上述结果表明,不同的CO2浓度可能对于植物免疫会产生不同的影响。
8 微生物组(microbiome) 8.1 根瘤菌、丛枝菌根真菌与植物免疫在豆科植物与根瘤菌的共生过程中,植物宿主需要抑制根瘤菌的肽聚糖和鞭毛蛋白等分子诱发的先天免疫反应以允许共生。因此,调节先天免疫的平衡对于根瘤菌的感染以及共生的建立与维持至关重要[171]。根瘤菌表面分子结构的突变(如脂多糖、环葡聚糖或胞外多糖EPS)可能导致共生缺陷,这表明天然根瘤菌多糖可能具有避免或抑制PTI的作用[172]。苜蓿根瘤菌(Sinorhizobium meliloti)与苜蓿属植物共生固氮的过程需要EPS,EPS可在共生早期阶段通过对抗H₂O₂发挥保护作用,从而帮助细菌抵御宿主的氧化胁迫[173]。研究表明,豆科植物百脉根中定位于细胞膜的蛋白激酶LjSymRK能够与LjBAK1相互作用,并抑制LjBAK1介导的植物免疫反应。Ljbak1突变体表现出比野生型更高水平的根瘤菌感染且不影响根瘤的发育,表明BAK1介导的抗性需要被削弱以实现共生[174]。
在丛枝菌根真菌(arbuscular mycorrhizal fungi, AMF)与植物的共生过程中,也需要克服植物的先天免疫来达到共生的目的。在AMF共生的早期,独脚金内酯(SLs)可诱导AMF真菌大量释放Myc因子(如短链几丁寡糖和非硫化脂质几丁寡糖),这些因子可被植物细胞膜上的LysM受体(如水稻中的CERK1和MYR1)感知,从而激活下游共生信号通路[175, 176]。为了建立共生关系,AMF会产生一些效应因子来抑制植物的防御反应[177]。例如,包含LysM的效应因子(secreted LysM-containing effector, SLM)能够和CO结合,通过保护真菌细胞壁免受几丁质酶的攻击,从而调节植物几丁质触发的免疫反应[178];效应因子SP7可通过抑制乙烯信号途径中的转录因子ERF19来降低植物免疫反应以促进共生[179];另一个定位于细胞核的效应因子NLE1,可通过干扰植物组蛋白H2B的泛素化来抑制植物防御相关基因的表达[180]。
植物激素是调控植物免疫和AMF共生的重要因子,在不同阶段通过协同和拮抗作用塑造植物的反应[181-183]。在共生的早期阶段,植物体内的SA水平短暂升高,用以激活防御相关的基因,如苯丙氨酸解氨酶基因PAL和PR基因[184]。在共生后期,不同植物中也观察到SA水平升高,可能用于限制根部过度定殖[185, 186]。此外,JA在共生根中也持续保持较高的水平,ABA、生长素和赤霉素的精细调控对AMF功能也有重要作用[181, 182, 187]。综上,AMF通过免疫抑制和激素调控来达到稳定共生的目的。
8.2 其他微生物与植物免疫植物体内部和周围栖居着数量庞大且种类繁多的微生物,构成了植物的微生物组。植物微生物组的物种组成和群落结构稳态能够有效抵御病原微生物对植物的侵染并维持植物健康,构成植物的“第二道免疫防线”[188, 189]。有益微生物组的保护作用一方面体现在通过拮抗或生态位竞争等方式直接抑制病原菌[190-194],另一方面微生物组中丰富的MAMPs有助于塑造植物形成完善的免疫系统。近年研究发现,在无菌种植体系下的拟南芥表现出PAMP触发植物PTI免疫的缺陷,部分拟南芥自发免疫突变体在无菌体系中丧失免疫表型,这些缺失的免疫响应都能在施加人工菌群后得以回补,表明了微生物组对植物免疫系统的重要调控作用[195, 196]。植物根际或叶际的许多微生物可以通过诱导防御相关基因表达、刺激胼胝质等防御相关次生代谢产物的分泌和累积、诱导植物挥发性化合物产生等方式[197-201],增强植物的诱导性系统抗性(induced systemic resistance, ISR)。而植物自身分泌的类黄酮类、香豆素类等化合物也能够富集有益微生物并促进其定殖,提高抵御病原菌的能力[200, 202-204]。
9 结论与讨论植物病理领域过去多年的工作多聚焦于植物与病原菌二者之间的互作,然而环境因素对于植物病害的影响是广泛而复杂的,这背后的机制研究还刚起步。不同环境因子可能通过病原菌的感知识别、免疫信号转导和下游免疫响应事件等不同的过程影响植物免疫反应,也可以通过改变病原菌的效应子产生、转运或者其他毒性因子的水平来调节病原菌侵染和定殖能力,这些作用综合起来决定了某一特定环境条件下病害的严重程度。解析环境因子对植物免疫和病原菌致病力的作用,不仅能为成功预测气候变化对植物病害的影响提供重要信息,还可为未来通过遗传学、基因工程、合成生物学等手段构建更具气候适应性的植物免疫系统提供关键理论基础。例如,部分研究中报道了决定植物免疫受体NLR蛋白对高温敏感性的特定氨基酸位点,通过对这些位点进行突变有助于获得在高温下仍具有功能的免疫蛋白[205];通过对拟南芥植物水杨酸合成相关的核心转录因子CBP60g进行元件改造,使其在高温下的转录不再受到抑制,从而使植物维持在高温下的水杨酸水平和抗病性[40]。此外,本文主要讨论的是单个环境因素对植物免疫系统或病原微生物的作用,但是在自然界中,植物经常面临多种环境胁迫的影响。多维环境因素的综合作用可能对宿主植物的生长性状和抗病性产生多态性的效果,探索其背后的机理将为改良作物应对复杂环境挑战提供重要的理论依据。
| [1] |
Stevens RB. Plant pathology, an advanced treatise: New York: Acadamic Press, 1960: 357-429.
|
| [2] |
Singh BK, Delgado-Baquerizo M, Egidi E, et al. Climate change impacts on plant pathogens, food security and paths forward. Nat Rev Microbiol, 2023, 21: 640-56. DOI:10.1038/s41579-023-00900-7 |
| [3] |
Raza MM, Bebber DP. Climate change and plant pathogens. Curr Opin Microbiol, 2022, 70: 102233. DOI:10.1016/j.mib.2022.102233 |
| [4] |
Wang Y, Pruitt RN, Nurnberger T, et al. Evasion of plant immunity by microbial pathogens. Nat Rev Microbiol, 2022, 20: 449-64. DOI:10.1038/s41579-022-00710-3 |
| [5] |
Bita C, Gerats T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci, 2013, 4: 273. |
| [6] |
Suzuki N, Rivero RM, Shulaev V, et al. Abiotic and biotic stress combinations. New Phytol, 2014, 203: 32-43. DOI:10.1111/nph.12797 |
| [7] |
Velasquez AC, Castroverde CDM, He SY. Plant-pathogen warfare under changing climate conditions. Curr Biol, 2018, 28: R619-34. DOI:10.1016/j.cub.2018.03.054 |
| [8] |
Pachauri RK, Meyer LA. Climate change 2014: synthesis report. IPCC: Geneva, 2014, 1059-72. |
| [9] |
Caffarra A, Rinaldi M, Eccel E, et al. Modelling the impact of climate change on the interaction between grapevine and its pests and pathogens: European grapevine moth and powdery mildew. Agr Ecosyst Environ, 2012, 148: 89-101. DOI:10.1016/j.agee.2011.11.017 |
| [10] |
Deutsch CA, Tewksbury JJ, Huey RB, et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci U S A, 2008, 105: 6668-72. DOI:10.1073/pnas.0709472105 |
| [11] |
Vaumourin E, Laine AL. Role of temperature and coinfection in mediating pathogen life-history traits. Front Plant Sci, 2018, 9: 1670. DOI:10.3389/fpls.2018.01670 |
| [12] |
Liu J, Feng L, Li J, et al. Genetic and epigenetic control of plant heat responses. Front Plant Sci, 2015, 6: 267. |
| [13] |
Nievola CC, Carvalho CP, Carvalho V, et al. Rapid responses of plants to temperature changes. Temperature (Austin), 2017, 4: 371-405. DOI:10.1080/23328940.2017.1377812 |
| [14] |
Gray SB, Brady SM. Plant developmental responses to climate change. Dev Biol, 2016, 419: 64-77. DOI:10.1016/j.ydbio.2016.07.023 |
| [15] |
Cheng C, Gao X, Feng B, et al. Plant immune response to pathogens differs with changing temperatures. Nat Commun, 2013, 4: 2530. DOI:10.1038/ncomms3530 |
| [16] |
Kumar SV, Wigge PA. H2A. Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell, 2010, 140: 136-47. DOI:10.1016/j.cell.2009.11.006 |
| [17] |
Huot B, Castroverde CDM, Velásquez AC, et al. Dual impact of elevated temperature on plant defence and bacterial virulence in Arabidopsis. Nat Commun, 2017, 8: 1808. DOI:10.1038/s41467-017-01674-2 |
| [18] |
Janda M, Lamparova L, Zubikova A, et al. Temporary heat stress suppresses PAMP-triggered immunity and resistance to bacteria in Arabidopsis thaliana. Mol Plant Pathol, 2019, 20: 1005-12. DOI:10.1111/mpp.12799 |
| [19] |
Menna A, Nguyen D, Guttman DS, et al. Elevated temperature differentially influences effector-triggered immunity outputs in Arabidopsis. Front Plant Sci, 2015, 6: 995. |
| [20] |
Wang Y, Bao Z, Zhu Y, et al. Analysis of temperature modulation of plant defense against biotrophic microbes. Mol Plant Microbe Interact, 2009, 22: 498-506. DOI:10.1094/MPMI-22-5-0498 |
| [21] |
Zhu Y, Qian W, Hua J. Temperature modulates plant defense responses through NB-LRR proteins. PLoS Pathog, 2010, 6: e1000844. DOI:10.1371/journal.ppat.1000844 |
| [22] |
Wang Z, Cui D, Liu J, et al. Arabidopsis ZED1-related kinases mediate the temperature-sensitive intersection of immune response and growth homeostasis. New Phytologist, 2017, 215: 711-24. DOI:10.1111/nph.14585 |
| [23] |
Wang Z, Cui D, Liu C, et al. TCP transcription factors interact with ZED1-related kinases as components of the temperature-regulated immunity. Plant Cell Environ, 2019, 42: 2045-56. DOI:10.1111/pce.13515 |
| [24] |
Webb KM, Ona I, Bai J, et al. A benefit of high temperature: increased effectiveness of a rice bacterial blight disease resistance gene. New Phytol, 2010, 185: 568-76. DOI:10.1111/j.1469-8137.2009.03076.x |
| [25] |
Jung JH, Domijan M, Klose C, et al. Phytochromes function as thermosensors in Arabidopsis. Science, 2016, 354: 886-9. DOI:10.1126/science.aaf6005 |
| [26] |
Legris M, Klose C, Burgie ES, et al. Phytochrome B integrates light and temperature signals in Arabidopsis. Science, 2016, 354: 897-900. DOI:10.1126/science.aaf5656 |
| [27] |
Gangappa SN, Berriri S, Kumar SV. PIF4 coordinates thermosensory growth and immunity in Arabidopsis. Curr Biol, 2017, 27: 243-9. DOI:10.1016/j.cub.2016.11.012 |
| [28] |
Hammoudi V, Fokkens L, Beerens B, et al. The Arabidopsis SUMO E3 ligase SIZ1 mediates the temperature dependent trade-off between plant immunity and growth. PLoS Genet, 2018, 14: e1007157. DOI:10.1371/journal.pgen.1007157 |
| [29] |
孟继彬. 小麦条锈病的发生及防治. 现代农业科技, 2019(07): 91. DOI:10.3969/j.issn.1007-5739.2019.07.053 |
| [30] |
Chen W, Wellings C, Chen X, et al. Wheat stripe (yellow) rust caused by Puccinia striiformis f. sp. tritici. Mol Plant Pathol, 2014, 15: 433-46. DOI:10.1111/mpp.12116 |
| [31] |
Wellings CR. Global status of stripe rust: a review of historical and current threats. Euphytica, 2011, 179: 129-41. DOI:10.1007/s10681-011-0360-y |
| [32] |
Beddow JM, Pardey PG, Chai Y, et al. Research investment implications of shifts in the global geography of wheat stripe rust. Nat Plants, 2015, 1: 15132. DOI:10.1038/nplants.2015.132 |
| [33] |
Wang J, Wang J, Li J, et al. The RLK protein TaCRK10 activates wheat high-temperature seedling-plant resistance to stripe rust through interacting with TaH2A. 1. Plant J, 2021, 108: 1241-55. DOI:10.1111/tpj.15513 |
| [34] |
Marques de Carvalho L, Benda ND, Vaughan MM, et al. Mi-1-mediated nematode resistance in tomatoes is broken by short-term heat stress but recovers over time. J Nematol, 2015, 47: 133-40. |
| [35] |
Moury B, Selassie KG, Marchoux G, et al. High temperature effects on hypersensitive resistance to tomato spotted wilt tospovirus (TSWV) in pepper (Capsicum chinense Jacq.). Eur J Plant Pathol, 1998, 104: 489-98. DOI:10.1023/A:1008618022144 |
| [36] |
Zhang W, Chen S, Abate Z, et al. Identification and characterization of Sr13, a tetraploid wheat gene that confers resistance to the Ug99 stem rust race group. Proc Natl Acad Sci U S A, 2017, 114: E9483-92. |
| [37] |
Chen S, Zhang W, Bolus S, et al. Identification and characterization of wheat stem rust resistance gene Sr21 effective against the Ug99 race group at high temperature. PLoS Genet, 2018, 14: e1007287. DOI:10.1371/journal.pgen.1007287 |
| [38] |
Richard MMS, Knip M, Aalders T, et al. Unlike many disease resistances, Rx1-mediated immunity to potato virus X is not compromised at elevated temperatures. Front Genet, 2020, 11: 417. DOI:10.3389/fgene.2020.00417 |
| [39] |
Rossi CAM, Marchetta EJR, Kim JH, et al. Molecular regulation of the salicylic acid hormone pathway in plants under changing environmental conditions. Trends Biochem Sci, 2023, 48: 699-712. DOI:10.1016/j.tibs.2023.05.004 |
| [40] |
Kim JH, Castroverde CDM, Huang S, et al. Increasing the resilience of plant immunity to a warming climate. Nature, 2022, 607: 339-44. DOI:10.1038/s41586-022-04902-y |
| [41] |
Huang S, Zhu S, Kumar P, et al. A phase-separated nuclear GBPL circuit controls immunity in plants. Nature, 2021, 594: 424-9. DOI:10.1038/s41586-021-03572-6 |
| [42] |
Samaradivakara SP, Chen H, Lu YJ, et al. Overexpression of NDR1 leads to pathogen resistance at elevated temperatures. New Phytol, 2022, 235: 1146-62. DOI:10.1111/nph.18190 |
| [43] |
Liu W, Liu J, Triplett L, et al. Novel insights into rice innate immunity against bacterial and fungal pathogens. Annu Rev Phytopathol, 2014, 52: 213-41. DOI:10.1146/annurev-phyto-102313-045926 |
| [44] |
Niño-Liu DO, Ronald PC, Bogdanove AJ. Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol Plant Pathol, 2006, 7: 303-24. DOI:10.1111/j.1364-3703.2006.00344.x |
| [45] |
陈贤, 汤宝, 赵延存, 等. 水稻细菌性条斑病的研究进展. 南京农业大学学报, 2025, 48: 46-57. DOI:10.7685/jnau.202405008 |
| [46] |
Cohen SP, Liu H, Argueso CT, et al. RNA-Seq analysis reveals insight into enhanced rice Xa7-mediated bacterial blight resistance at high temperature. PLoS One, 2017, 12: e0187625. DOI:10.1371/journal.pone.0187625 |
| [47] |
Dean R, Van Kan JA, Pretorius ZA, et al. The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol, 2012, 13: 414-30. DOI:10.1111/j.1364-3703.2011.00783.x |
| [48] |
Qiu J, Xie J, Chen Y, et al. Warm temperature compromises JA-regulated basal resistance to enhance Magnaporthe oryzae infection in rice. Mol Plant, 2022, 15: 723-39. DOI:10.1016/j.molp.2022.02.014 |
| [49] |
Wang J, Tian W, Tao F, et al. TaRPM1 positively regulates wheat high-temperature seedling-plant resistance to Puccinia striiformis f. sp. tritici. Front Plant Sci, 2019(10): 1679. |
| [50] |
Hu Y, Tao F, Su C, et al. NBS-LRR gene TaRPS2 is positively associated with the high-temperature seedling plant resistance of wheat against Puccinia striiformis f. sp. tritici. Phytopathology, 2021, 111: 1449-58. DOI:10.1094/PHYTO-03-20-0063-R |
| [51] |
Hasegawa H, Chatterjee A, Cui Y, et al. Elevated temperature enhances virulence of Erwinia carotovora subsp. carotovora strain EC153 to plants and stimulates production of the quorum sensing signal, N-acyl homoserine lactone, and extracellular proteins. Appl Environ Microbiol, 2005, 71: 4655-63. DOI:10.1128/AEM.71.8.4655-4663.2005 |
| [52] |
Jin S, Song YN, Deng WY, et al. The regulatory VirA protein of Agrobacterium tumefaciens does not function at elevated temperatures. J Bacteriol, 1993, 175: 6830-5. DOI:10.1128/jb.175.21.6830-6835.1993 |
| [53] |
Baron C, Domke N, Beinhofer M, et al. Elevated temperature differentially affects virulence, VirB protein accumulation, and T-Pilus formation in different Agrobacterium tumefaciens and Agrobacterium vitis strains. J Bacteriol, 2001, 183: 6852-61. DOI:10.1128/JB.183.23.6852-6861.2001 |
| [54] |
van Dijk K, Fouts DE, Rehm AH, et al. The Avr (effector) proteins HrmA (HopPsyA) and AvrPto are secreted in culture from Pseudomonas syringae pathovars via the Hrp (type Ⅲ) protein secretion system in a temperature- and pH-sensitive manner. J Bacteriol, 1999, 181: 4790-7. DOI:10.1128/JB.181.16.4790-4797.1999 |
| [55] |
Li Z, Liu H, Ding Z, et al. Low Temperature enhances plant immunity via salicylic acid pathway genes that are repressed by ethylene. Plant Physiol, 2020, 182: 626-39. DOI:10.1104/pp.19.01130 |
| [56] |
Leach MD, Cowen LE. Surviving the heat of the moment: a fungal pathogens perspective. PLoS Pathog, 2013, 9: e1003163. DOI:10.1371/journal.ppat.1003163 |
| [57] |
Xiao W, Zhang J, Huang J, et al. Response and regulatory mechanisms of heat resistance in pathogenic fungi. Appl Microbiol Biotechnol, 2022, 106: 5415-31. DOI:10.1007/s00253-022-12119-2 |
| [58] |
刘天华, 白姣姣, 吕东平. 农业气象因素影响稻瘟病发生分子机制初探. 中国生态农业学报(中英文), 2016, 24: 1-7. |
| [59] |
Bui DC, Lee Y, Lim JY, et al. Heat shock protein 90 is required for sexual and asexual development, virulence, and heat shock response in Fusarium graminearum. Sci Rep, 2016, 6: 28154. DOI:10.1038/srep28154 |
| [60] |
Wang Y, Kerns JP. Temperature effects on formation of appressoria and sporulation of Colletotrichum cereale on two turfgrass species. Inter Turfgrass Soc Res J, 2017, 13: 123-32. DOI:10.2134/itsrj2016.06.0451 |
| [61] |
Sabburg R, Obanor F, Aitken E, et al. Changing fitness of a necrotrophic plant pathogen under increasing temperature. Glob Chang Biol, 2015, 21: 3126-37. DOI:10.1111/gcb.12927 |
| [62] |
Cheng S, Wang S, Li M, et al. Summer heatwaves in China during 1961-2021: the impact of humidity. Atmos Res, 2024, 304: 107366. DOI:10.1016/j.atmosres.2024.107366 |
| [63] |
Guo J, Chen S. Long-term change in relative humidity across China from 1961-2018. Clim Res, 2022, 87: 167-81. DOI:10.3354/cr01691 |
| [64] |
Cheng V, Ng E, Chan C, et al. Outdoor thermal comfort study in a sub-tropical climate: a longitudinal study based in Hong Kong. Int J Biometeorol, 2012, 56: 43-56. DOI:10.1007/s00484-010-0396-z |
| [65] |
Granke LL, Hausbeck MK. Effects of temperature, humidity, and wounding on development of phytophthora rot of cucumber fruit. Plant Dis, 2010, 94: 1417-24. DOI:10.1094/PDIS-04-10-0258 |
| [66] |
Panchal S, Chitrakar R, Thompson BK, et al. Regulation of stomatal defense by air relative humidity. Plant Physiol, 2016, 172: 2021-32. DOI:10.1104/pp.16.00696 |
| [67] |
Yao L, Jiang Z, Wang Y, et al. High air humidity dampens salicylic acid pathway and NPR1 function to promote plant disease. EMBO J, 2023, 42: e113499. DOI:10.15252/embj.2023113499 |
| [68] |
Melotto M, Underwood W, Koczan J, et al. Plant stomata function in innate immunity against bacterial invasion. Cell, 2006, 126: 969-80. DOI:10.1016/j.cell.2006.06.054 |
| [69] |
Paauw M, van Hulten M, Chatterjee S, et al. Hydathode immunity protects the Arabidopsis leaf vasculature against colonization by bacterial pathogens. Curr Biol, 2023, 33: 697-710. DOI:10.1016/j.cub.2023.01.013 |
| [70] |
Qiu J, Liu Z, Xie J, et al. Dual impact of ambient humidity on the virulence of Magnaporthe oryzae and basal resistance in rice. Plant Cell Environ, 2022, 45: 3399-411. DOI:10.1111/pce.14452 |
| [71] |
Jiang Z, Yao L, Zhu X, et al. Ethylene signaling modulates air humidity responses in plants. Plant J, 2024, 117: 653-68. DOI:10.1111/tpj.16556 |
| [72] |
Jambunathan N, Siani JM, McNellis TW. A humidity-sensitive Arabidopsis copine mutant exhibits precocious cell death and increased disease resistance. Plant Cell, 2001, 13: 2225-40. DOI:10.1105/tpc.010226 |
| [73] |
Zhou F, Menke FL, Yoshioka K, et al. High humidity suppresses ssi4-mediated cell death and disease resistance upstream of MAP kinase activation, H2O2 production and defense gene expression. Plant J, 2004, 39: 920-32. DOI:10.1111/j.1365-313X.2004.02180.x |
| [74] |
Wang C, Cai X, Zheng Z. High humidity represses Cf-4/Avr4- and Cf-9/Avr9-dependent hypersensitive cell death and defense gene expression. Planta, 2005, 222: 947-56. DOI:10.1007/s00425-005-0036-8 |
| [75] |
Fall ML, Van der Heyden H, Beaulieu C, et al. Bremia lactucae infection efficiency in lettuce is modulated by temperature and leaf wetness duration under quebec field conditions. Plant Dis, 2015, 99: 1010-9. DOI:10.1094/PDIS-05-14-0548-RE |
| [76] |
Peng Z, Hu Y, Zhang J, et al. Xanthomonas translucens commandeers the host rate-limiting step in ABA biosynthesis for disease susceptibility. Proc Natl Acad Sci U S A, 2019, 116: 20938-46. DOI:10.1073/pnas.1911660116 |
| [77] |
Hu Y, Ding Y, Cai B, et al. Bacterial effectors manipulate plant abscisic acid signaling for creation of an aqueous apoplast. Cell Host Microbe, 2022, 30: 518-29. DOI:10.1016/j.chom.2022.02.002 |
| [78] |
Roussin-Léveillée C, Lajeunesse G, St-Amand M, et al. Evolutionarily conserved bacterial effectors hijack abscisic acid signaling to induce an aqueous environment in the apoplast. Cell Host Microbe, 2022, 30: 489-501. DOI:10.1016/j.chom.2022.02.006 |
| [79] |
Nomura K, Andreazza F, Cheng J, et al. Bacterial pathogens deliver water- and solute-permeable channels to plant cells. Nature, 2023, 621: 586-91. DOI:10.1038/s41586-023-06531-5 |
| [80] |
Lam HN, Chakravarthy S, Wei HL, et al. Global analysis of the HrpL regulon in the plant pathogen Pseudomonas syringae pv. tomato DC3000 reveals new regulon members with diverse functions. PLoS One, 2014, 9: e106115. DOI:10.1371/journal.pone.0106115 |
| [81] |
Manstretta V, Rossi V. Effects of temperature and moisture on development of Fusarium graminearum perithecia in maize stalk residues. Appl Environ Microbiol, 2016, 82: 184-91. DOI:10.1128/AEM.02436-15 |
| [82] |
Leplat J, Friberg H, Abid M, et al. Survival of Fusarium graminearum, the causal agent of Fusarium head blight. Agron Sustain Dev, 2012, 33: 97-111. |
| [83] |
Nnadi NE, Carter DA. Climate change and the emergence of fungal pathogens. PLoS Pathog, 2021, 17: e1009503. DOI:10.1371/journal.ppat.1009503 |
| [84] |
Baptista FJ, Bailey BJ, Meneses JF. Effect of nocturnal ventilation on the occurrence of Botrytis cinerea in Mediterranean unheated tomato greenhouses. Crop Prot, 2012, 32: 144-9. DOI:10.1016/j.cropro.2011.11.005 |
| [85] |
Clarkson JP, Fawcett L, Anthony SG, et al. A model for Sclerotinia sclerotiorum infection and disease development in lettuce, based on the effects of temperature, relative humidity and ascospore density. PLoS One, 2014, 9: e94049. DOI:10.1371/journal.pone.0094049 |
| [86] |
Wallenhammar AC, Omer ZS, Edin E, et al. Influence of soil-borne inoculum of Plasmodiophora brassicae measured by qPCR on disease severity of clubroot-resistant cultivars of winter oilseed rape (Brassica napus L.). Pathogens, 2021, 10: 433. DOI:10.3390/pathogens10040433 |
| [87] |
Cheng MC, Kathare PK, Paik I, et al. Phytochrome signaling networks. Annu Rev Plant Biol, 2021, 72: 217-44. DOI:10.1146/annurev-arplant-080620-024221 |
| [88] |
Ballare CL. Light regulation of plant defense. Annu Rev Plant Biol, 2014, 65: 335-63. DOI:10.1146/annurev-arplant-050213-040145 |
| [89] |
Pierik R, Ballare CL. Control of plant growth and defense by photoreceptors: from mechanisms to opportunities in agriculture. Mol Plant, 2021, 14: 61-76. DOI:10.1016/j.molp.2020.11.021 |
| [90] |
de Wit M, Spoel SH, Sanchez-Perez GF, et al. Perception of low red: far-red ratio compromises both salicylic acid- and jasmonic acid-dependent pathogen defences in Arabidopsis. Plant J, 2013, 75: 90-103. DOI:10.1111/tpj.12203 |
| [91] |
Genoud T, Buchala AJ, Chua NH, et al. Phytochrome signalling modulates the SA-perceptive pathway in Arabidopsis. Plant J, 2002, 31: 87-95. DOI:10.1046/j.1365-313X.2002.01338.x |
| [92] |
Griebel T, Zeier J. Light regulation and daytime dependency of inducible plant defenses in Arabidopsis: phytochrome signaling controls systemic acquired resistance rather than local defense. Plant Physiol, 2008, 147: 790-801. DOI:10.1104/pp.108.119503 |
| [93] |
Yang YX, Wang MM, Yin YL, et al. RNA-seq analysis reveals the role of red light in resistance against Pseudomonas syringae pv. tomato DC3000 in tomato plants. BMC Genomics, 2015, 16: 120. DOI:10.1186/s12864-015-1228-7 |
| [94] |
Yuan P, Yang S, Feng L, et al. Red-light receptor phytochrome B inhibits BZR1-NAC028-CAD8B signaling to negatively regulate rice resistance to sheath blight. Plant Cell Environ, 2023, 46: 1249-63. DOI:10.1111/pce.14502 |
| [95] |
Li X, Chen H, Yang S, et al. Phytochrome B promotes blast disease resistance and enhances yield in rice. Plant Physiol, 2024, 196: 3023-32. DOI:10.1093/plphys/kiae509 |
| [96] |
Wu L, Yang HQ. CRYPTOCHROME 1 is implicated in promoting R protein-mediated plant resistance to Pseudomonas syringae in Arabidopsis. Mol Plant, 2010, 3: 539-48. DOI:10.1093/mp/ssp107 |
| [97] |
Hao Y, Zeng Z, Yuan M, et al. The blue-light receptor CRY1 serves as a switch to balance photosynthesis and plant defense. Cell Host Microbe, 2025, 33: 137-50. DOI:10.1016/j.chom.2024.12.003 |
| [98] |
Naqvi S, He Q, Trusch F, et al. Blue-light receptor phototropin 1 suppresses immunity to promote Phytophthora infestans infection. New Phytol, 2022, 233: 2282-93. DOI:10.1111/nph.17929 |
| [99] |
Lin T, Qiu H, Cheng D, et al. Potato NPH3/RPT2-like (NRL) member StNRL-9 interacts with Stphots and negatively regulates late blight resistance. Physiol Plant, 2024, 176: e14594. DOI:10.1111/ppl.14594 |
| [100] |
Lajeunesse G, Roussin-Leveillee C, Boutin S, et al. Light prevents pathogen-induced aqueous microenvironments via potentiation of salicylic acid signaling. Nat Commun, 2023, 14: 713. DOI:10.1038/s41467-023-36382-7 |
| [101] |
Huang H, Nguyen Thi Thu T, He X, et al. Increase of fungal pathogenicity and role of plant glutamine in nitrogen-induced susceptibility (NIS) to rice blast. Front Plant Sci, 2017, 8: 265. |
| [102] |
Ballini E, Nguyen TT, Morel JB. Diversity and genetics of nitrogen-induced susceptibility to the blast fungus in rice and wheat. Rice, 2013, 6: 32. DOI:10.1186/1939-8433-6-32 |
| [103] |
车喜庆, 李振宇, 邢亚楠, 等. 氮肥运筹对盐粳系列水稻品种(系)抗瘟性评价及稻瘟病对稻米品质的影响. 北方水稻, 2023, 53: 10-3+9. |
| [104] |
Luo C, Ma L, Zhu J, et al. Effects of nitrogen and intercropping on the occurrence of wheat powdery mildew and stripe rust and the relationship with crop yield. Front Plant Sci, 2021, 12: 637393. DOI:10.3389/fpls.2021.637393 |
| [105] |
Lecompte F, Abro MA, Nicot PC. Can plant sugars mediate the effect of nitrogen fertilization on lettuce susceptibility to two necrotrophic pathogens: Botrytis cinerea and Sclerotinia sclerotiorum?. Plant Soil, 2013, 369: 387-401. DOI:10.1007/s11104-012-1577-9 |
| [106] |
Hoffland E, van Beusichem ML, Jeger MJ. Nitrogen availability and susceptibility of tomato leaves to Botrytis cinerea. Plant Soil, 1999, 210: 263-72. DOI:10.1023/A:1004661913224 |
| [107] |
Vega A, Canessa P, Hoppe G, et al. Transcriptome analysis reveals regulatory networks underlying differential susceptibility to Botrytis cinerea in response to nitrogen availability in Solanum lycopersicum. Front Plant Sci, 2015, 6: 911. |
| [108] |
Dutta A, Dracatos PM, Khan GA. Balancing act: the dynamic relationship between nutrient availability and plant defence. Plant J, 2024, 120: 1724-34. DOI:10.1111/tpj.17098 |
| [109] |
Camargo EL, Nascimento LC, Soler M, et al. Contrasting nitrogen fertilization treatments impact xylem gene expression and secondary cell wall lignification in Eucalyptus. BMC Plant Biol, 2014, 14: 256. DOI:10.1186/s12870-014-0256-9 |
| [110] |
Plavcova L, Hacke UG, Almeida-Rodriguez AM, et al. Gene expression patterns underlying changes in xylem structure and function in response to increased nitrogen availability in hybrid poplar. Plant Cell Environ, 2013, 36: 186-99. DOI:10.1111/j.1365-3040.2012.02566.x |
| [111] |
Yaeno T, Iba K. BAH1/NLA, a RING-type ubiquitin E3 ligase, regulates the accumulation of salicylic acid and immune responses to Pseudomonas syringae DC3000. Plant Physiol, 2008, 148: 1032-41. DOI:10.1104/pp.108.124529 |
| [112] |
Ding S, Shao X, Li J, et al. Nitrogen forms and metabolism affect plant defence to foliar and root pathogens in tomato. Plant Cell Environ, 2021, 44: 1596-610. DOI:10.1111/pce.14019 |
| [113] |
Camanes G, Pastor V, Cerezo M, et al. A deletion in NRT2.1 attenuates Pseudomonas syringae-induced hormonal perturbation, resulting in primed plant defenses. Plant Physiol, 2012, 158: 1054-66. DOI:10.1104/pp.111.184424 |
| [114] |
Dechorgnat J, Patrit O, Krapp A, et al. Characterization of the Nrt2.6 gene in Arabidopsis thaliana: a link with plant response to biotic and abiotic stress. PLoS One, 2012, 7: e42491. DOI:10.1371/journal.pone.0042491 |
| [115] |
Modolo LV, Augusto O, Almeida IM, et al. Nitrite as the major source of nitric oxide production by Arabidopsis thaliana in response to Pseudomonas syringae. FEBS Lett, 2005, 579: 3814-20. DOI:10.1016/j.febslet.2005.05.078 |
| [116] |
Modolo LV, Augusto O, Almeida IMG, et al. Decreased arginine and nitrite levels in nitrate reductase-deficient Arabidopsis thaliana plants impair nitric oxide synthesis and the hypersensitive response to Pseudomonas syringae. Plant Sci, 2006, 171: 34-40. DOI:10.1016/j.plantsci.2006.02.010 |
| [117] |
Tabata R, Sumida K, Yoshii T, et al. Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science, 2014, 346: 343-6. DOI:10.1126/science.1257800 |
| [118] |
Ohkubo Y, Tanaka M, Tabata R, et al. Shoot-to-root mobile polypeptides involved in systemic regulation of nitrogen acquisition. Nat Plants, 2017, 3: 17029. DOI:10.1038/nplants.2017.29 |
| [119] |
Rzemieniewski J, Leicher H, Lee HK, et al. CEP signaling coordinates plant immunity with nitrogen status. Nat Commun, 2024, 15: 10686. DOI:10.1038/s41467-024-55194-x |
| [120] |
Farjad M, Clément G, Launay A, et al. Plant nitrate supply regulates Erwinia amylovora virulence gene expression in Arabidopsis. Mol Plant Pathol, 2021, 22: 1332-46. DOI:10.1111/mpp.13114 |
| [121] |
Pérez-García A, Snoeijers SS, Joosten MH, et al. Expression of the avirulence gene Avr9 of the fungal tomato pathogen Cladosporium fulvum is regulated by the global nitrogen response factor NRF1. Mol Plant Microbe Interact, 2001, 14: 316-25. DOI:10.1094/MPMI.2001.14.3.316 |
| [122] |
Hinsinger P, Betencourt E, Bernard L, et al. P for two, sharing a scarce resource: soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiol, 2011, 156: 1078-86. DOI:10.1104/pp.111.175331 |
| [123] |
Mudge SR, Rae AL, Diatloff E, et al. Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J, 2002, 31: 341-53. DOI:10.1046/j.1365-313X.2002.01356.x |
| [124] |
Duan K, Yi K, Dang L, et al. Characterization of a sub-family of Arabidopsis genes with the SPX domain reveals their diverse functions in plant tolerance to phosphorus starvation. Plant J, 2008, 54: 965-75. DOI:10.1111/j.1365-313X.2008.03460.x |
| [125] |
Nilsson L, Muller R, Nielsen TH. Increased expression of the MYB-related transcription factor, PHR1, leads to enhanced phosphate uptake in Arabidopsis thaliana. Plant Cell Environ, 2007, 30: 1499-512. DOI:10.1111/j.1365-3040.2007.01734.x |
| [126] |
Chan C, Liao YY, Chiou TJ. The impact of phosphorus on plant immunity. Plant Cell Physiol, 2021, 62: 582-9. DOI:10.1093/pcp/pcaa168 |
| [127] |
Campos-Soriano L, Bundo M, Bach-Pages M, et al. Phosphate excess increases susceptibility to pathogen infection in rice. Mol Plant Pathol, 2020, 21: 555-70. DOI:10.1111/mpp.12916 |
| [128] |
Jaskolowski A, Poirier Y. Phosphate deficiency increases plant susceptibility to Botrytis cinerea infection by inducing the abscisic acid pathway. Plant J, 2024, 119: 828-43. DOI:10.1111/tpj.16800 |
| [129] |
Luo X, Li Z, Xiao S, et al. Phosphate deficiency enhances cotton resistance to Verticillium dahliae through activating jasmonic acid biosynthesis and phenylpropanoid pathway. Plant Sci, 2021, 302: 110724. DOI:10.1016/j.plantsci.2020.110724 |
| [130] |
Khan GA, Vogiatzaki E, Glauser G, et al. Phosphate deficiency induces the jasmonate pathway and enhances resistance to insect herbivory. Plant Physiol, 2016, 171: 632-44. DOI:10.1104/pp.16.00278 |
| [131] |
Dong Z, Li W, Liu J, et al. The rice phosphate transporter protein OsPT8 regulates disease resistance and plant growth. Sci Rep, 2019, 9: 5408. DOI:10.1038/s41598-019-41718-9 |
| [132] |
Tang J, Wu D, Li X, et al. Plant immunity suppression via PHR1-RALF-FERONIA shapes the root microbiome to alleviate phosphate starvation. EMBO J, 2022, 41: e109102. DOI:10.15252/embj.2021109102 |
| [133] |
He K, Du J, Han X, et al. PHOSPHATE STARVATION RESPONSE1 (PHR1) interacts with JASMONATE ZIM-DOMAIN (JAZ) and MYC2 to modulate phosphate deficiency-induced jasmonate signaling in Arabidopsis. Plant Cell, 2023, 35: 2132-56. DOI:10.1093/plcell/koad057 |
| [134] |
Dindas J, DeFalco TA, Yu G, et al. Direct inhibition of phosphate transport by immune signaling in Arabidopsis. Curr Biol, 2022, 32: 488-95. DOI:10.1016/j.cub.2021.11.063 |
| [135] |
Martin-Cardoso H, Bundo M, Val-Torregrosa B, et al. Phosphate accumulation in rice leaves promotes fungal pathogenicity and represses host immune responses during pathogen infection. Front Plant Sci, 2023, 14: 1330349. |
| [136] |
Liu G, Greenshields DL, Sammynaiken R, et al. Targeted alterations in iron homeostasis underlie plant defense responses. J Cell Sci, 2007, 120: 596-605. DOI:10.1242/jcs.001362 |
| [137] |
Dangol S, Chen Y, Hwang BK, et al. Iron- and reactive oxygen species-dependent ferroptotic cell death in rice-Magnaporthe oryzae interactions. Plant Cell, 2019, 31: 189-209. DOI:10.1105/tpc.18.00535 |
| [138] |
Sánchez-Sanuy F, Mateluna-Cuadra R, Tomita K, et al. Iron induces resistance against the rice blast fungus Magnaporthe oryzae through potentiation of immune responses. Rice, 2022, 15: 68. DOI:10.1186/s12284-022-00609-w |
| [139] |
Ye F, Albarouki E, Lingam B, et al. An adequate Fe nutritional status of maize suppresses infection and biotrophic growth of Colletotrichum graminicola. Physiol Plantarum, 2014, 151: 280-92. DOI:10.1111/ppl.12166 |
| [140] |
Aznar A, Chen NW, Rigault M, et al. Scavenging iron: a novel mechanism of plant immunity activation by microbial siderophores. Plant Physiol, 2014, 164: 2167-83. DOI:10.1104/pp.113.233585 |
| [141] |
Platre MP, Satbhai SB, Brent L, et al. The receptor kinase SRF3 coordinates iron-level and flagellin dependent defense and growth responses in plants. Nat Commun, 2022, 13: 4445. DOI:10.1038/s41467-022-32167-6 |
| [142] |
Cao M, Platre MP, Tsai HH, et al. Spatial IMA1 regulation restricts root iron acquisition on MAMP perception. Nature, 2024, 625: 750-9. DOI:10.1038/s41586-023-06891-y |
| [143] |
Segond D, Dellagi A, Lanquar V, et al. NRAMP genes function in Arabidopsis thaliana resistance to Erwinia chrysanthemi infection. Plant J, 2009, 58: 195-207. DOI:10.1111/j.1365-313X.2008.03775.x |
| [144] |
Dellagi A, Rigault M, Segond D, et al. Siderophore‐mediated upregulation of Arabidopsis ferritin expression in response to Erwinia chrysanthemi infection. Plant J, 2005, 43: 262-72. DOI:10.1111/j.1365-313X.2005.02451.x |
| [145] |
Nguyen NK, Wang J, Liu D, et al. Rice iron storage protein ferritin 2 (OsFER2) positively regulates ferroptotic cell death and defense responses against Magnaporthe oryzae. Front Plant Sci, 2022, 13: 1019669. DOI:10.3389/fpls.2022.1019669 |
| [146] |
Liu Y, Kong D, Wu HL, et al. Iron in plant-pathogen interactions. J Exp Bot, 2020, 72: 2114-24. |
| [147] |
Xing Y, Xu N, Bhandari DD, et al. Bacterial effector targeting of a plant iron sensor facilitates iron acquisition and pathogen colonization. Plant Cell, 2021, 33: 2015-31. DOI:10.1093/plcell/koab075 |
| [148] |
Hsu FC, Chou MY, Chou SJ, et al. Submergence confers immunity mediated by the WRKY22 transcription factor in Arabidopsis. Plant Cell, 2013, 25: 2699-713. DOI:10.1105/tpc.113.114447 |
| [149] |
Mooney BC, Doorly CM, Mantz M, et al. Hypoxia represses pattern-triggered immune responses in Arabidopsis. Plant Physiol, 2024, 196: 2064-77. DOI:10.1093/plphys/kiae432 |
| [150] |
Burgess T, McComb JA, Colquhoun I, et al. Increased susceptibility of Eucalyptus marginata to stem infection by Phytophthora cinnamomi resulting from root hypoxia. Plant Pathol, 1999, 48: 797-806. DOI:10.1046/j.1365-3059.1999.00396.x |
| [151] |
Babujee L, Apodaca J, Balakrishnan V, et al. Evolution of the metabolic and regulatory networks associated with oxygen availability in two phytopathogenic enterobacteria. BMC Genomics, 2012, 13: 110. DOI:10.1186/1471-2164-13-110 |
| [152] |
McDonald KL, Sutherland MW, Guest DI. Temporary hypoxia suppresses the oxidative burst and subsequent hypersensitive cell death in cells of tobacco and soybean challenged with zoospores of incompatible isolates of Phytophthora species. Physiol Mol Plant Pathol, 2002, 61: 133-40. DOI:10.1006/pmpp.2002.0423 |
| [153] |
De Boer SH, Kelman A. Influence of oxygen concentration and storage factors on susceptibility of potato tubers to bacterial soft rot (Erwinia carotovora). Potato Res, 1978, 21: 65-79. DOI:10.1007/BF02362262 |
| [154] |
Charkowski AO. The changing face of bacterial soft-rot diseases. Annu Rev Phytopathol, 2018, 56: 269-88. DOI:10.1146/annurev-phyto-080417-045906 |
| [155] |
Lisicka W, Fikowicz-Krosko J, Jafra S, et al. Oxygen availability influences expression of Dickeya solani genes associated with virulence in potato (Solanum tuberosum L.). Front Plant Sci, 2018, 9: 374. DOI:10.3389/fpls.2018.00374 |
| [156] |
Moslemi A, Ades PK, Groom T, et al. Influence of waterlogging on growth of pyrethrum plants infected by the crown and root rot pathogens, Fusarium oxysporum, Fusarium avenaceum and Paraphoma vinacea. Australas Plant Pathol, 2018, 47: 205-13. DOI:10.1007/s13313-018-0547-y |
| [157] |
Valeri MC, Novi G, Weits DA, et al. Botrytis cinerea induces local hypoxia in Arabidopsis leaves. New Phytol, 2021, 229: 173-85. DOI:10.1111/nph.16513 |
| [158] |
Camy C, Dreyer E, Delatour C, et al. Responses of the root rot fungus Collybia fusipes to soil waterlogging and oxygen availability. Mycol Res, 2003, 107: 1103-9. DOI:10.1017/S095375620300830X |
| [159] |
Chung H, Kim S, Kim KT, et al. A novel approach to investigate hypoxic microenvironment during rice colonization by Magnaporthe oryzae. Environ Microbiol, 2019, 21: 1151-69. DOI:10.1111/1462-2920.14563 |
| [160] |
Johansen TJ, Dees MW, Hermansen A. High soil moisture reduces common scab caused by Streptomyces turgidiscabies and Streptomyces europaeiscabiei in potato. Acta Agr Scand B-s P, 2014, 65: 193-8. |
| [161] |
Bidzinski P, Ballini E, Ducasse A, et al. Transcriptional basis of drought-induced susceptibility to the rice blast fungus Magnaporthe oryzae. Front Plant Sci, 2016, 7: 1558. |
| [162] |
Irulappan V, Kandpal M, Saini K, et al. Drought stress exacerbates fungal colonization and endodermal invasion and dampens defense responses to increase dry root rot in chickpea. Mol Plant Microbe Interact, 2022, 35: 583-91. DOI:10.1094/MPMI-07-21-0195-FI |
| [163] |
Moeder W, Ung H, Mosher S, et al. SA-ABA antagonism in defense responses. Plant Signal Behav, 2010, 5: 1231-3. DOI:10.4161/psb.5.10.12836 |
| [164] |
Choudhary A, Senthil-Kumar M. Drought attenuates plant defence against bacterial pathogens by suppressing the expression of CBP60g/SARD1 during combined stress. Plant Cell Environ, 2022, 45: 1127-45. DOI:10.1111/pce.14275 |
| [165] |
Gupta A, Senthil-Kumar M. Transcriptome changes in Arabidopsis thaliana infected with Pseudomonas syringae during drought recovery. Sci Rep, 2017, 7: 9124. DOI:10.1038/s41598-017-09135-y |
| [166] |
Martinez-Ferri E, Moreno-Ortega G, van den Berg N, et al. Mild water stress-induced priming enhance tolerance to Rosellinia necatrix in susceptible avocado rootstocks. BMC Plant Biol, 2019, 19: 458. DOI:10.1186/s12870-019-2016-3 |
| [167] |
Zhang S, Li X, Sun Z, et al. Antagonism between phytohormone signalling underlies the variation in disease susceptibility of tomato plants under elevated CO2. J Exp Bot, 2015, 66: 1951-63. DOI:10.1093/jxb/eru538 |
| [168] |
Hu Z, Sun Z, Ma Q, et al. N-decanoyl-homoserine lactone alleviates elevated CO2-induced defense suppression to Botrytis cinerea in tomato. Sci Hortic, 2020, 268: 109353. DOI:10.1016/j.scienta.2020.109353 |
| [169] |
Zhou Y, Vroegop-Vos I, Schuurink RC, et al. Atmospheric CO2 alters resistance of Arabidopsis to Pseudomonas syringae by affecting abscisic acid accumulation and stomatal responsiveness to coronatine. Front Plant Sci, 2017, 8: 700. DOI:10.3389/fpls.2017.00700 |
| [170] |
Mhamdi A, Noctor G. High CO2 primes plant biotic stress defences through redox-linked pathways. Plant Physiol, 2016, 172: 929-42. |
| [171] |
Gourion B, Berrabah F, Ratet P, et al. Rhizobium-legume symbioses: the crucial role of plant immunity. Trends Plant Sci, 2015, 20: 186-94. DOI:10.1016/j.tplants.2014.11.008 |
| [172] |
Jones KM, Kobayashi H, Davies BW, et al. How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model. Nat Rev Microbiol, 2007, 5: 619-33. DOI:10.1038/nrmicro1705 |
| [173] |
Lehman AP, Long SR. Exopolysaccharides from Sinorhizobium meliloti can protect against H2O2-dependent damage. J Bacteriol, 2013, 195: 5362-9. DOI:10.1128/JB.00681-13 |
| [174] |
Feng Y, Wu P, Liu C, et al. Suppression of LjBAK1-mediated immunity by SymRK promotes rhizobial infection in Lotus japonicus. Mol Plant, 2021, 14: 1935-50. DOI:10.1016/j.molp.2021.07.016 |
| [175] |
He J, Zhang C, Dai H, et al. A LysM receptor heteromer mediates perception of arbuscular mycorrhizal symbiotic signal in rice. Mol Plant, 2019, 12: 1561-76. DOI:10.1016/j.molp.2019.10.015 |
| [176] |
Shi J, Wang X, Wang E. Mycorrhizal symbiosis in plant growth and stress adaptation: from genes to ecosystems. Annu Rev Plant Biol, 2023, 74: 569-607. DOI:10.1146/annurev-arplant-061722-090342 |
| [177] |
Lo Presti L, Lanver D, Schweizer G, et al. Fungal effectors and plant susceptibility. Annu Rev Plant Biol, 2015, 66: 513-45. DOI:10.1146/annurev-arplant-043014-114623 |
| [178] |
Zeng T, Rodriguez-Moreno L, Mansurkhodzaev A, et al. A lysin motif effector subverts chitin-triggered immunity to facilitate arbuscular mycorrhizal symbiosis. New Phytol, 2020, 225: 448-60. DOI:10.1111/nph.16245 |
| [179] |
Kloppholz S, Kuhn H, Requena N. A secreted fungal effector of Glomus intraradices promotes symbiotic biotrophy. Curr Biol, 2011, 21: 1204-9. DOI:10.1016/j.cub.2011.06.044 |
| [180] |
Wang P, Jiang H, Boeren S, et al. A nuclear-targeted effector of Rhizophagus irregularis interferes with histone 2B mono‐ubiquitination to promote arbuscular mycorrhization. New Phytol, 2021, 230: 1142-55. DOI:10.1111/nph.17236 |
| [181] |
Ho-Plágaro T, García-Garrido JM. Molecular regulation of arbuscular mycorrhizal symbiosis. Int J Mol Sci, 2022, 23: 5960. DOI:10.3390/ijms23115960 |
| [182] |
Liao D, Wang S, Cui M, et al. Phytohormones regulate the development of arbuscular mycorrhizal symbiosis. Int J Mol Sci, 2018, 19: 3146. DOI:10.3390/ijms19103146 |
| [183] |
Pozo MJ, López-Ráez JA, Azcón-Aguilar C, et al. Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol, 2015, 205: 1431-6. DOI:10.1111/nph.13252 |
| [184] |
Blilou I, Ocampo JA, García‐Garrido JM. Induction of Ltp (lipid transfer protein) and Pal (phenylalanine ammonia-lyase) gene expression in rice roots colonized by the arbuscular mycorrhizal fungus Glomus mosseae. J Exp Bot, 2000, 51: 1969-77. DOI:10.1093/jexbot/51.353.1969 |
| [185] |
Fujita M, Kusajima M, Fukagawa M, et al. Response of tomatoes primed by mycorrhizal colonization to virulent and avirulent bacterial pathogens. Sci Rep, 2022, 12: 4686. DOI:10.1038/s41598-022-08395-7 |
| [186] |
Pozo MJ, Azcón-Aguilar C. Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol, 2007, 10: 393-8. DOI:10.1016/j.pbi.2007.05.004 |
| [187] |
Fernández I, Merlos M, López-Ráez J, et al. Defense related phytohormones regulation in arbuscular mycorrhizal symbioses depends on the partner genotypes. J Chem Ecol, 2014, 40: 791-803. DOI:10.1007/s10886-014-0473-6 |
| [188] |
Vogel CM, Potthoff DB, Schafer M, et al. Protective role of the Arabidopsis leaf microbiota against a bacterial pathogen. Nat Microbiol, 2021, 6: 1537-48. DOI:10.1038/s41564-021-00997-7 |
| [189] |
Duran P, Thiergart T, Garrido-Oter R, et al. Microbial interkingdom interactions in roots promote Arabidopsis survival. Cell, 2018, 175: 973-83. DOI:10.1016/j.cell.2018.10.020 |
| [190] |
Xu S, Liu YX, Cernava T, et al. Fusarium fruiting body microbiome member Pantoea agglomerans inhibits fungal pathogenesis by targeting lipid rafts. Nat Microbiol, 2022, 7: 831-43. DOI:10.1038/s41564-022-01131-x |
| [191] |
Kwak MJ, Kong HG, Choi K, et al. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat Biotechnol, 2018. DOI:10.1038/nbt.4232 |
| [192] |
Chen Y, Wang J, Yang N, et al. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat Commun, 2018, 9: 3429. DOI:10.1038/s41467-018-05683-7 |
| [193] |
Innerebner G, Knief C, Vorholt JA. Protection of Arabidopsis thaliana against leaf-pathogenic Pseudomonas syringae by Sphingomonas strains in a controlled model system. Appl Environ Microbiol, 2011, 77: 3202-10. DOI:10.1128/AEM.00133-11 |
| [194] |
Schafer M, Pacheco AR, Kunzler R, et al. Metabolic interaction models recapitulate leaf microbiota ecology. Science, 2023, 381: eadf5121. DOI:10.1126/science.adf5121 |
| [195] |
Cheng YT, Thireault CA, Zhang L, et al. Roles of microbiota in autoimmunity in Arabidopsis leaves. Nat Plants, 2024, 10: 1363-76. DOI:10.1038/s41477-024-01779-9 |
| [196] |
Paasch BC, Sohrabi R, Kremer JM, et al. A critical role of a eubiotic microbiota in gating proper immunocompetence in Arabidopsis. Nat Plants, 2023, 9: 1468-80. DOI:10.1038/s41477-023-01501-1 |
| [197] |
Kong HG, Song GC, Sim HJ, et al. Achieving similar root microbiota composition in neighbouring plants through airborne signalling. ISME J, 2021, 15: 397-408. DOI:10.1038/s41396-020-00759-z |
| [198] |
Zhou F, Emonet A, Denervaud Tendon V, et al. Co-incidence of damage and microbial patterns controls localized immune responses in roots. Cell, 2020, 180: 440-53. DOI:10.1016/j.cell.2020.01.013 |
| [199] |
Chialva M, Salvioli di Fossalunga A, Daghino S, et al. Native soils with their microbiotas elicit a state of alert in tomato plants. New Phytol, 2018, 220: 1296-308. DOI:10.1111/nph.15014 |
| [200] |
Liu X, Matsumoto H, Lv T, et al. Phyllosphere microbiome induces host metabolic defence against rice false-smut disease. Nat Microbiol, 2023, 8: 1419-33. DOI:10.1038/s41564-023-01379-x |
| [201] |
Cellini A, Buriani G, Rocchi L, et al. Biological relevance of volatile organic compounds emitted during the pathogenic interactions between apple plants and Erwinia amylovora. Mol Plant Pathol, 2018, 19: 158-68. DOI:10.1111/mpp.12509 |
| [202] |
Stringlis IA, Yu K, Feussner K, et al. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc Natl Acad Sci U S A, 2018, 115: E5213-22. |
| [203] |
Hassan S, Mathesius U. The role of flavonoids in root-rhizosphere signalling: opportunities and challenges for improving plant-microbe interactions. J Exp Bot, 2012, 63: 3429-44. DOI:10.1093/jxb/err430 |
| [204] |
Kulkarni OS, Mazumder M, Kini S, et al. Volatile methyl jasmonate from roots triggers host-beneficial soil microbiome biofilms. Nat Chem Biol, 2024, 20: 473-83. DOI:10.1038/s41589-023-01462-8 |
| [205] |
Venkatesh J, Kang BC. Current views on temperature-modulated R gene-mediated plant defense responses and tradeoffs between plant growth and immunity. Curr Opin Plant Biol, 2019, 50: 9-17. DOI:10.1016/j.pbi.2019.02.002 |
 2025, Vol. 37
2025, Vol. 37 

 辛秀芳,研究员、课题组长、博士生导师。2014年博士毕业于美国密歇根州立大学,2017年加入中国科学院分子植物科学卓越创新中心,同期加入中国科学院-英国约翰.英纳斯中心(John Innes Centre) 植物与微生物科学联合研究中心。长期从事“植物与微生物互作”研究,在Nature、Cell Host & Microbe、EMBO Journal等国际主流学术期刊发表多篇研究文章。获得2023年国家自然科学基金委杰出青年、2023年国际分子植物与微生物互作学会颁发的早期职业成就奖、中国科学院青年科学家奖等。现任Journal of Integrative Plant Biology杂志编委、中国植物生理与植物分子生物学学会植物微生物分子互作专业委员会副主任
辛秀芳,研究员、课题组长、博士生导师。2014年博士毕业于美国密歇根州立大学,2017年加入中国科学院分子植物科学卓越创新中心,同期加入中国科学院-英国约翰.英纳斯中心(John Innes Centre) 植物与微生物科学联合研究中心。长期从事“植物与微生物互作”研究,在Nature、Cell Host & Microbe、EMBO Journal等国际主流学术期刊发表多篇研究文章。获得2023年国家自然科学基金委杰出青年、2023年国际分子植物与微生物互作学会颁发的早期职业成就奖、中国科学院青年科学家奖等。现任Journal of Integrative Plant Biology杂志编委、中国植物生理与植物分子生物学学会植物微生物分子互作专业委员会副主任