(2 浙江工业大学药学院,杭州 310014)
(2 College of Pharmacy, Zhejiang University of Technology, Hangzhou 310014, China)
牛磺酸又称β-氨基乙磺酸,是一种含硫的条件必需氨基酸,在生物体内主要以游离形式存在。其通过TauT转运蛋白转运入胞,在哺乳动物心脏、脑及肝脏等高代谢器官中富集,通过调节细胞渗透压、稳定膜结构及介导蛋白质磷酸化等机制维持细胞稳态,并参与胆汁酸结合等关键代谢过程。牛磺酸通过激活自噬通路、抑制氧化应激等作用,在改善神经退行性疾病及延缓衰老进程中有显著效果。本综述总结了近年来牛磺酸在抗衰老方面的研究进展,讨论了利用合成生物学策略以益生菌为底盘细胞合成牛磺酸的优势,以更好地应用牛磺酸改善代谢,延长最大寿命和健康寿命。
1 牛磺酸与抗衰老牛磺酸的研究历程可追溯至19世纪:1827年,Tiedemann等[1]首次从牛胆汁中分离出牛磺酸,并命名为Gallen-Asparagin;1838年,Taurin一词首次出现于Demarcay等[2]发表的文章中,并沿用至今。然而,受限于早期技术手段,其生理功能直至近四十年才被系统阐明:牛磺酸不仅参与胆汁酸代谢、离子稳态调控等生理过程,更在抗衰老(图 1)、神经保护及代谢疾病干预中发挥重要作用。牛磺酸通过调控自噬、抗氧化应激等方式延缓组织退变的分子通路已成为当前研究热点。
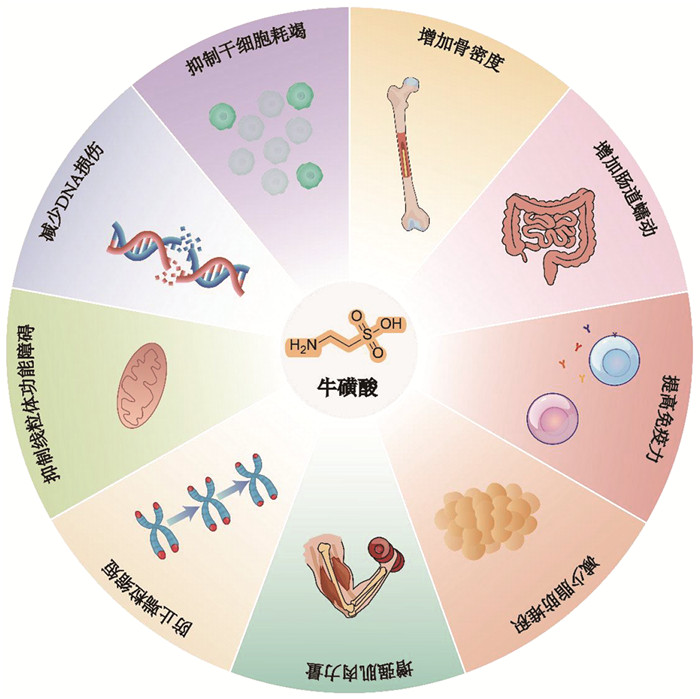
|
图 1 牛磺酸改善衰老相关指标 |
机体衰老是一个复杂的生物学过程,涉及组织器官、细胞、遗传、表观遗传等不同层面的系统性变化。机体牛磺酸水平与衰老发展的进程密切相关[3, 4]。1986年,Ommati等[5]研究发现长期低牛磺酸饮食可导致血清牛磺酸含量降低20%,在老年群体中这一现象更加明显,血清牛磺酸含量降低达80%[4, 5],提示牛磺酸的内源性合成能力可能随年龄增长而大幅下降。2023年,Singh等[4]发现机体牛磺酸水平随年龄增长显著下降,而外源性补充可逆转多物种衰老进程:使线虫寿命延长10%~ 23%,小鼠寿命延长10%~12%,并促进免疫系统与器官年轻化,改善衰老相关指标[6]。牛磺酸抗衰老作用的机制,可归纳为三个方面:(1)通过清除自由基、激活抗氧化酶系(如SOD、谷胱甘肽)抵抗氧化应激;(2)通过调控代谢通路(如H2S信号、SIRT1-p53轴)维持能量与代谢稳态;(3)通过抑制神经炎症、保护心血管功能延缓组织退行性病变。以下将围绕这三个方面系统阐述牛磺酸的多维度抗衰老机制。
1.1 抗氧化应激作用牛磺酸作为多功能抗氧化剂,其抗衰老功能的核心在于通过调控氧化应激反应以延缓细胞损伤与组织退化[7]。并且,牛磺酸的抗氧化作用对线粒体功能完整性的保持有积极贡献[8, 9]。
牛磺酸最直接的抗氧化作用体现在清除次氯酸(HClO)等剧毒氧化剂,通过消耗髓过氧化物酶(myeloperoxidase, MPO)-卤化物系统产生的次氯酸阻断其对细胞的氧化损伤[10]。在骨骼肌衰老方面,Barbiera等[11]通过腹膜内给药证实,牛磺酸可以显著降低炎症因子,改善肌肉微环境中的氧化还原平衡,不仅抵消衰老相关的再生障碍,还能通过维持组织稳态延缓骨骼肌退行性变化过程。在骨关节系统中,牛磺酸通过O-GlcNAc糖基转移酶(O-GlcNAc transferase, OGT)介导的O-GlcNAc糖基化修饰与谷胱甘肽过氧化物酶4 (glutathione peroxidase 4, GPX4)依赖性抗铁死亡途径,协同抑制软骨细胞氧化损伤并延缓退行性病变[12]。针对衰老相关代谢性疾病,其对肝脏的保护作用表现为激活Nrf2信号轴,提升超氧化物歧化酶(superoxide dismutase, SOD)、过氧化氢酶(catalase, CAT)等抗氧化酶活性及下游血红素加氧酶-1 (HEME oxygenase-1, HO-1)、NAD(P)H:醌氧化还原酶1(NAD(P)H:quinone oxi-doreductase 1, NQO1)等分子表达,同时抑制JAK2-STAT1、TLR4/NF-κB等促炎通路,形成氧化-炎症双调控网络[13, 14];而在高脂高糖饮食诱导的胰腺β细胞中,通过Nrf2/HO-1通路增强抗氧化能力,同时抑制线粒体凋亡,有效对抗高糖脂毒性[15]。对于环境毒素引发的衰老相关损伤,牛磺酸不仅能与辅酶Q10协同逆转异丙肾上腺素导致的肝肾氧化应激[16],还可通过降低活性氧(reactive oxygen species, ROS)、丙二醛(malon-dialdehyde, MDA)等标志物,阻断NF-κB介导的炎症级联反应及线粒体凋亡途径,缓解霉菌毒素DON引发的肝损伤[7]。值得注意的是,牛磺酸对物理性损伤(如放疗)和生物性应激(如水产氨暴露)同样展现普适性抗氧化效应:在放射性颌骨修复中减少DNA损伤并促进骨再生[17];对大黄鱼(Larimichthys crocea)则通过提升SOD/GPx活性及溶菌酶功能,将MDA水平降低35.3%,有效维持氧化-免疫稳态[18]。人类临床研究进一步验证其抗衰老潜力,55~70岁女性补充牛磺酸16周后,血浆SOD水平显著提升且MDA增长被抑制,证实其通过维持抗氧化酶活性对抗衰老相关的氧化失衡[19]。
这些跨物种、多组织的抗氧化机制共同揭示,牛磺酸通过维持氧化还原平衡、阻断炎症、调控程序性死亡通路等核心环节,系统性延缓氧化应激驱动的细胞衰老进程,为抗衰老干预提供了多靶点策略。
1.2 调节代谢稳态除了发挥抗氧化应激作用,牛磺酸还通过多维度机制协同维持代谢稳态以发挥抗衰老作用:在细胞层面,其通过增强谷胱甘肽抗氧化防御体系、抑制铁过载、促进甘油磷脂代谢修复细胞膜,并提升线粒体能量代谢,全面改善氧化还原稳态,从而抑制铁死亡并维持骨骼肌细胞的代谢完整性和再生能力[20];在器官互作层面,牛磺酸差异化调控肝脏(受膳食成分调节)与肠道(受能量供应主导)的代谢酶表达,维持两器官间代谢协同平衡[21],同时通过定向富集于白色脂肪组织、上调合成关键酶(如半胱氨酸双加氧酶)及转运蛋白TauT表达,协同肠道菌群与胆汁酸代谢促进脂肪分解,并介导肝脏-肠道-微生物群-脂肪组织的多器官代谢信号联动,从而在热量限制条件下调节代谢稳态以驱动脂肪代谢并维持能量平衡[22];2021年,Tang等[23]研究发现,在饮食限制的43名健康受试者中,血清牛磺酸在禁食第三天较禁食前增加了31%~46%,并与肠道菌群形成“牛磺酸-微生物”调节环路维持糖脂代谢稳态,并且可通过调控AMPK/mTOR磷酸化级联抑制感染诱导的代谢通路过度激活[24];此外,其与胆汁酸结合形成的牛磺胆酸不仅促进胆固醇转化、改善脂质吸收以减少肝脏脂肪蓄积[25-27],还通过抑制炎性介质(如一氧化氮、前列腺素E2、组胺)发挥系统性抗炎作用[28],从而多途径延缓代谢紊乱相关的衰老进程。
1.3 神经与心血管保护作用牛磺酸有显著的神经保护和心血管保护作用。在神经保护方面,牛磺酸通过减少氧化应激、抑制神经胶质激活、维持突触连接及促进视网膜色素上皮吞噬功能,显著减缓RCS大鼠光感受器退化并增强视网膜功能,表现出神经保护作用[29]。另外,牛磺酸通过抑制炎症因子(TNF-α、IL-6)释放、减少α-突触核蛋白聚集、提升多巴胺水平,有效缓解帕金森样症状和神经退行性损伤[30, 31]。其神经保护作用还包括调节小胶质细胞活化[31, 32]、维持突触可塑性[32]、促进神经营养因子表达[33]、减轻血脑屏障损伤和铁死亡等[34]。在阿尔茨海默病模型中,牛磺酸通过上调髓样细胞触发受体2 (triggering receptor expressed on myeloid cells 2, TREM2)的表达抑制小胶质细胞过度活化[35],同时改善记忆障碍并调节下丘脑-垂体-肾上腺轴(hypothalamic-pituitary- adrenal axis, HPA or HTPA axis)功能[33],从而改善认知功能,缓解病情,展现其神经保护作用。此外,牛磺酸还能有效对抗药物(MK-801)引发的记忆缺陷和运动过度问题,让斑马鱼在实验中保持正常记忆和活动状态,说明它能通过保护大脑功能来缓解类似精神分裂症的症状,为未来治疗提供新思路[36]。
在心血管系统方面,祁增华等[37, 38]通过老年小鼠双盲实验模拟高污染暴露,补充牛磺酸可显著缓解PM2.5暴露引发的老年小鼠心脏损伤。此外,牛磺酸可通过激活SIRT1-p53通路改善心脏功能(提升NAD+/NADH比值、抑制心肌纤维化和氧化应激)[39];通过TonE/TonEBP信号轴上调TauT表达以抵御心肌缺血损伤[40];并通过CBS-H2S通路改善营养不良相关的血管内皮功能障碍[41]。这些机制共同表明,牛磺酸通过协同抗氧化、抗炎、抗凋亡、调节代谢和突触功能等途径,在神经退行性疾病和心血管衰老中发挥关键保护作用,为抗衰老干预提供了重要的分子靶点和治疗策略。
1.4 牛磺酸的其他生理功能牛磺酸作为广泛分布于哺乳动物组织的条件必需氨基酸,参与多种细胞活动,调控细胞稳态。在渗透压平衡中,其通过调节Na+/Cl-协同摄取,动态调节细胞内离子梯度,进而发挥渗透压调节的作用[42-44]。在膜稳定性方面,牛磺酸通过静电作用优化磷脂比例,抑制磷脂酶C活性[45],增加钙结合位点密度[46],并促进钙泵和钠钾泵功能[47],从而增强膜稳定性。在信号转导中,牛磺酸可抑制JNK磷酸化、ROS生成和环氧化酶-2 (cyclooxygenase-2, COX-2)表达,从而发挥抗破骨细胞生成作用[48]。此外,在抗肿瘤方面,牛磺酸通过下调N-钙黏蛋白(N-cadherin, CDH2)、扭转蛋白1 (Twist-related protein 1, TWIST1)等上皮-间质转化标志物并上调上皮钙黏蛋白(E-cadherin, E-Cad)[49],抑制乳腺癌、结直肠癌等增殖转移,并在动物模型中显著降低AOM/DSS诱导的结肠癌发生率[50, 51]。
2 牛磺酸的生物代谢与合成截至2022年,牛磺酸的全球市场规模约为15亿美元,预计到2030年将达到25亿美元,2024— 2030年的复合年增长率为6.5%。市场增长主要得益于功能饮料(如红牛)和宠物食品需求的持续攀升,以及医药领域(心血管疾病治疗)的应用扩展[52]。
目前牛磺酸的工业化生产方法包括化学合成法、酶解法,以及从动物材料中直接提取。化学合成法是当前的主流方法,但存在高能耗、环境污染等问题。基于角蛋白原料的生物酶解法,需依赖特定酶催化体系,成本较高。从动物材料(海鲜、软体动物、鱿鱼、水生生物等)中直接提取天然牛磺酸,占总牛磺酸市场份额的比例较小(材料有限),而且产物易残留动物组织成分,存在药用安全性隐患[53]。随着合成生物学的发展,利用合成生物学技术理念,以肠道益生菌为底盘细胞,在肠道原位合成牛磺酸以弥补机体衰老导致的牛磺酸合成能力不足,增加牛磺酸的供应,改善衰老及老龄化相关疾病,是一个极具商业价值的研究方向。
2.1 牛磺酸在真核细胞中的合成牛磺酸的生物合成主要有三条途径:(1)由L-半胱氨酸经半胱氨酸双加氧酶(cysteine dioxygenase, CDO)和半胱氨酸亚磺酸脱羧酶(cysteine sulfinic acid decarboxylase, CSAD)催化形成亚牛磺酸,再由含黄素单加氧酶1 (flavin containing monooxygenase 1, FMO1)催化氧合形成牛磺酸;(2)由L-半胱氨酸经CDO直接催化形成磺基丙氨酸,再由谷氨酸脱羧酶样蛋白1 (glutamate decarboxylase like protein 1, GADL1)和FMO1催化形成牛磺酸;(3)由半胱胺通过半胱胺双加氧酶(cysteamine dioxygenase, ADO)催化形成亚牛磺酸并通过FMO1介导转化为牛磺酸(图 2)。牛磺酸在肠道中被牛磺酸呼吸细菌代谢,主要代谢产物有牛磺胆酸盐、脒基牛磺酸和N-乙酰牛磺酸等。
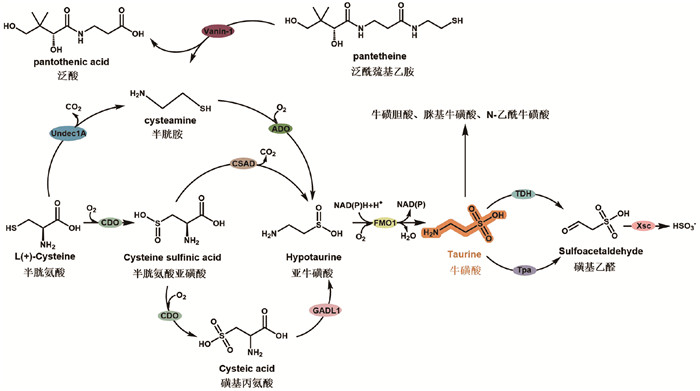
|
图 2 牛磺酸的生物代谢 |
牛磺酸在血浆、胆汁、唾液和肌肉组织(心肌、骨骼肌)中非常丰富,心脏中牛磺酸浓度可达6 μmol/L,血浆牛磺酸浓度可达50~150 μmol/L [54, 55]。其合成代谢途径(图 2)在哺乳动物中已经得到充分研究,主要以L-半胱氨酸为底物,经CDO氧化形成L-半胱氨酸亚磺酸,进而在CSAD的作用下,脱羧形成亚牛磺酸,亚牛磺酸被FMO1氧化形成牛磺酸[56-60]。半胱氨酸也可经CDO两步氧化形成磺基丙氨酸,再由CSAD或GADL1脱羧为亚牛磺酸[61],进而转化成牛磺酸。乙酰辅酶A在细胞内可被水解为泛酰巯基乙胺,泛酰巯基乙胺进一步通过泛酰巯基乙胺酶(Vanin-1)催化,生成泛酸和半胱胺,同时为半胱氨酸脱羧形成半胱胺提供能量,半胱胺在ADO的催化作用下形成亚牛磺酸[62, 63]。亚牛磺酸也可自发形成牛磺酸。
2.2 牛磺酸在原核细胞中的合成在原核生物中牛磺酸的合成研究相对较少。在枯草芽孢杆菌(B. subtilis 168)中,半胱氨酸通过CDO转化为半胱氨酸亚磺酸,虽然其CDO与哺乳动物CDO的基因序列仅有15%~30%的相似度,但其活性位点高度保守[64],说明细菌中也存在与哺乳动物类似的牛磺酸生物合成途径。2017年,邓洁等[65]通过未培养土壤微生物宏基因组文库分析得到的L-半胱氨酸亚磺酸脱羧酶Undec1A,能够催化L-半胱氨酸脱羧生成β-巯基乙胺,即半胱胺[66, 67]。此外,2013年,Agnello等[56]通过对结构及序列高度相关的人源CSAD (hCSAD)与谷氨酸脱羧酶(hGAD)进行对比分析,鉴定出一个三残基底物识别基序(X1aa19X2aaX3),该基序通过活性位点的空间排布特异性结合不同底物。通过引入该基序,可以将人类谷氨酸脱羧酶(hGAD)改造成对半胱氨酸亚磺酸具有选择性的酶,并且该基序被发现在海洋细菌(如Synechococcus sp. PCC 7335)中存在,揭示了原核生物中牛磺酸的生物合成途径,表明这种氨基酸的产生不仅限于真核生物,而是分布在整个生命树中。
2.3 牛磺酸的合成生物学细胞工厂鉴于牛磺酸在抗衰老方面的益处,开发高效合成牛磺酸的益生菌前景广阔。构建基于合成生物学的牛磺酸细胞工厂,智能化响应宿主体内牛磺酸水平、动态合成和分泌牛磺酸,实时定量补充衰老过程中缺失的牛磺酸,最终实现改善衰老和延长健康寿命的目的。目前,利用合成生物学技术手段,在改造底盘菌生产牛磺酸方面已取得一定进展。利用代谢工程手段在谷氨酸棒状杆菌中引入半胱氨酸合酶(cysteate synthase, CS)、CDO1、CSAD的编码基因,结合代谢调控改造,构建了牛磺酸工程菌株Tau11,24 h内产量达62.0±2.4 mg/g [68];将鲤鱼(C.carpio)来源的CDO和CSAD编码基因异源整合到莱茵衣藻(C.reinhardtii)叶绿体中,也成功实现了牛磺酸的合成,产量为0.13~0.14 mg/g [69]。然而,仍存在一些技术瓶颈,比如:底盘菌株与人体肠道微环境适配性不足,难以长时间在肠道存活;牛磺酸合成中间产物可能对宿主细胞产生毒性,限制高产菌株的构建;缺乏牛磺酸相关的合成生物学元件,牛磺酸的智能化响应系统尚未见报道;牛磺酸的主效受体蛋白也尚未鉴定。亟需挖掘更加高效的牛磺酸合成元件与牛磺酸智能化响应元件,利用合成生物学技术手段在益生菌底盘细胞中优化重构牛磺酸合成与分泌系统,以活体益生菌形式在肠道中原位合成与分泌牛磺酸,从而发挥抗衰老的效果,实现延长寿命同时改善老龄化相关疾病的目的。
2.4 牛磺酸的分解代谢牛磺酸是含硫氨基酸的最终代谢产物[70],在哺乳动物中,牛磺酸下游的几种次级代谢物分别包括牛磺胆酸盐、脒基牛磺酸和N-乙酰牛磺酸。胆酸辅酶A:氨基酸N-酰基转移酶(bile acid-CoA: amino acid N-acyltransferase, BAAT)将牛磺酸与胆汁酸结合生成牛磺胆酸盐和其他胆汁盐。除此之外,2024年,Wei等[71]在哺乳动物鉴定到首个N-乙酰牛磺酸水解酶——PTER,其能够将N-乙酰牛磺酸水解为牛磺酸和乙酸酯,并且催化牛磺酸向N-乙酰牛磺酸的方向转变。而在微生物中,牛磺酸通过牛磺酸脱氢酶(tauropine dehydrogenase, TDH)形成磺基乙醛,该酶需要细胞色素C维持活性,或者通过牛磺酸-丙酮酸转氨酶(taurine-pyruvate aminotransferase, Tpa)催化形成磺基乙醛;然后在磺乙醛乙酰转移酶(sulfoacetaldehyde acetyltransferase, Xsc)的作用下,牛磺酸脱磺生成亚硫酸盐[57, 72-75]。
3 总结及展望牛磺酸具有多种生理功能,包括抗衰老、抗氧化、维持渗透压和膜稳定性等。随着年龄的增长,机体内牛磺酸含量逐渐降低,这一变化与多种衰老相关表型的出现密切相关,但最近也有研究表明,牛磺酸浓度不随年龄单纯减少,存在较大个体差异,且存在种属差异,因此定义牛磺酸为一种衰老标志物还有待深入研究[76]。外源性补充牛磺酸被证明能够显著抑制这些衰老表型变化,从而改善组织功能并延长健康寿命。
基于合成生物学技术手段,以肠道益生菌为底盘细胞,构建智能化响应机体牛磺酸含量的工程益生菌,使其能够实时动态监测机体牛磺酸含量、动态且适量地合成牛磺酸,弥补由老龄化导致的机体牛磺酸水平下降,从而发挥抗衰老及改善老龄代谢疾病的作用,是一种极具商业前景的新型抗衰老干预策略,主要方法包括:(1)建立个性化牛磺酸需求预测模型,实现人体牛磺酸含量水平的精准预测;(2)开发牛磺酸浓度传感器,构建牛磺酸含量水平的智能化监测系统;(3)开发牛磺酸合成和分泌的工程益生菌,实现牛磺酸含量的精准动态控制。
| [1] |
Tiedemann F, Gmelin L. Einige neue bestandtheile der galle des ochsen. AdP, 1827, 85: 326-37. |
| [2] |
Demarcay H. Ueber die natur der Galle. J Prakt Chem, 1838, 15: 193-212. |
| [3] |
Marcinkiewicz J, Kontny E. Taurine and inflammatory diseases. Amino acids, 2014, 46: 7-20. |
| [4] |
Singh P, Gollapalli K, Mangiola S, et al. Taurine deficiency as a driver of aging. Science, 2023, 380: eabn9257. |
| [5] |
Ommati MM, Rezaei H, Socorro RM, et al. Pre/postnatal taurine supplementation improves neurodevelopment and brain function in mice offspring: a persistent developmental study from puberty to maturity. Life Sci, 2024, 336: 122284. |
| [6] |
Izquierdo JM. Taurine as a possible therapy for immuno-senescence and inflammaging. Cell Mol Immunol, 2024, 21: 3-5. |
| [7] |
Ji X, Tang Z, Zhang F, et al. Dietary taurine supplementation counteracts deoxynivalenol-induced liver injury via alleviating oxidative stress, mitochondrial dysfunction, apoptosis, and inflammation in piglets. Ecotoxicol Environ Saf, 2023, 253: 114705. |
| [8] |
Jong CJ, Azuma J, Schaffer S. Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids, 2012, 42: 2223-32. |
| [9] |
Surai PF, Earle-Payne K, Kidd MT. Taurine as a natural antioxidant: from direct antioxidant effects to protective action in various toxicological models. Antioxidants (Basel), 2021, 10: 1876. |
| [10] |
Weiss SJ, Klein R, Slivka A, et al. Chlorination of taurine by human neutrophils: evidence for hypochlorous acid generation. J Clin Invest, 1982, 70: 598-607. |
| [11] |
Barbiera A, Sorrentino S, Fard D, et al. Taurine administration counteracts aging-associated impingement of skeletal muscle regeneration by reducing inflammation and oxidative stress. Antioxidants (Basel), 2022, 11: 1016. |
| [12] |
Zhou X, Yang Y, Qiu X, et al. Antioxidant taurine inhibits chondrocyte ferroptosis through upregulation of OGT/Gpx4 signaling in osteoarthritis induced by anterior cruciate ligament transection. J Adv Res, 2025, S2090-1232(25)00029-3. |
| [13] |
Ouyang G, Wang N, Tong J, et al. Alleviation of taurine on liver injury of type 2 diabetic rats by improving antioxidant and anti-inflammatory capacity. Heliyon, 2024, 10: e28400. |
| [14] |
Shi Y, Zhong L, Fan Y, et al. Taurine inhibits hydrogen peroxide-induced oxidative stress, inflammatory response and apoptosis in liver of Monopterus albus. Fish Shellfish Immunol, 2022, 128: 536-46. |
| [15] |
Zhao D, Yin D, Wang X, et al. Taurine alleviates high-fat-high-glucose-induced pancreatic islet β-cell oxidative stress and apoptosis in rat. Heliyon, 2023, 9: e21879. |
| [16] |
Moke EG, Asiwe JN, Ben-Azu B, et al. Co-enzyme-Q10 and taurine abate isoprenaline-mediated hepatorenal dysregulations and oxidative stress in rats. Clin Nutr Open Sci, 2024, 57: 10-25. |
| [17] |
Chen H, Zheng M, Li M, et al. Taurine ameliorates radiation-induced oxidative stress in bone marrow mesenchymal stromal cells and promotes osteogenesis. Free Radic Biol Med, 2024, 225: 805-20. |
| [18] |
Zhao X, Xu Z, Mei J, et al. Taurine inhibits acute ammonia-induced oxidative stress, immune response, and apoptosis of large yellow croaker (Larimichthys crocea) during keep-live transport. J Agr Food Res, 2025, 19: 101716. |
| [19] |
Abud GF, De Carvalho FG, Batitucci G, et al. Taurine as a possible antiaging therapy: a controlled clinical trial on taurine antioxidant activity in women ages 55 to 70. Nutrition, 2022, 101: 111706. |
| [20] |
Liu X, Zhou Y, Qi Z, et al. Taurine alleviates ferroptosis-induced metabolic impairments in C2C12 myoblasts by stabilizing the labile iron pool and improving redox homeostasis. J Proteome Res, 2024, 23: 3444-59. |
| [21] |
Gregor A, Auñon-Lopez A, Pignitter M, et al. The distinct mechanism regulating taurine homeostasis in mice: nutrient availability affects taurine levels in the liver and energy restriction influences it in the intestine. Life Sci, 2024, 359: 123213. |
| [22] |
Sarra F, Paocic D, Zöchling A, et al. Gut microbiota, dietary taurine, and fiber shift taurine homeostasis in adipose tissue of calorie-restricted mice to impact fat loss. J Nutr Biochem, 2024, 134: 109720. |
| [23] |
Tang L, Li L, Bu L, et al. Bigu-style fasting affects metabolic health by modulating taurine, glucose, and cholesterol homeostasis in healthy young adults. J Nutr, 2021, 151: 2175-87. |
| [24] |
蓝日国. 牛磺酸通过AMPK-mTOR调节能量代谢缓解乳房链球菌感染诱导的炎症[D]. 南京: 南京农业大学, 2021
|
| [25] |
Song Q, Guo J, Zhang Y, et al. The beneficial effects of taurine in alleviating fatty liver disease. J Funct Foods, 2021, 77: 104351. |
| [26] |
Falany CN, Johnson MR, Barnes S, et al. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA: amino acid N-acyltransferase. J Biol Chem, 1994, 269: 19375-9. |
| [27] |
Murakami S, Fujita M, Nakamura M, et al. Taurine ameliorates cholesterol metabolism by stimulating bile acid production in high-cholesterol-fed rats. Clin Exp Pharmacol Physiol, 2016, 43: 372-8. |
| [28] |
Guizoni DM, Vettorazzi JF, Carneiro EM, et al. Modulation of endothelium-derived nitric oxide production and activity by taurine and taurine-conjugated bile acids. Nitric Oxide, 2020, 94: 48-53. |
| [29] |
Martínez-Vacas A, Di Pierdomenico J, Gallego-Ortega A, et al. Systemic taurine treatment affords functional and morphological neuroprotection of photoreceptors and restores retinal pigment epithelium function in RCS rats. Redox Biol, 2022, 57: 102506. |
| [30] |
Onuelu JE, Ben-Azu B, Adebayo OG, et al. Taurine, an essential amino acid, attenuates rotenone-induced Parkinson's disease in rats by inhibiting α-synuclein aggregation and augmenting dopamine release. Behav Brain Res, 2025, 480: 115397. |
| [31] |
Che Y, Hou L, Sun F, et al. Taurine protects dopaminergic neurons in a mouse Parkinson's disease model through inhibition of microglial M1 polarization. Cell Death Dis, 2018, 9: 435. |
| [32] |
Wang K, Shi Y, Liu W, et al. Taurine improves neuron injuries and cognitive impairment in a mouse Parkinson's disease model through inhibition of microglial activation. Neurotoxicology, 2021, 83: 129-36. |
| [33] |
Wu GF, Ren S, Tang RY, et al. Antidepressant effect of taurine in chronic unpredictable mild stress-induced depressive rats. Sci Rep, 2017, 7: 4989. |
| [34] |
Liu C, He P, Guo Y, et al. Taurine attenuates neuronal ferroptosis by regulating GABAB/AKT/GSK3β/β-catenin pathway after subarachnoid hemorrhage. Free Radic Biol Med, 2022, 193: 795-807. |
| [35] |
Ahmed S, Ma N, Kawanokuchi J, et al. Taurine reduces microglia activation in the brain of aged senescence-accelerated mice by increasing the level of TREM2. Sci Rep, 2024, 14: 7427. |
| [36] |
Franscescon F, Müller TE, Bertoncello KT, et al. Neuroprotective role of taurine on MK-801-induced memory impairment and hyperlocomotion in zebrafish. Neurochem Int, 2020, 135: 104710. |
| [37] |
Qi Z, Yang C, Liao X, et al. Taurine reduction associated with heart dysfunction after real-world PM2.5 exposure in aged mice. Sci Total Environ, 2021, 782: 146866. |
| [38] |
Yang S, Wen L, Chai X, et al. The protective effects of taurine and fish oil supplementation on PM2.5-induced heart dysfunction among aged mice: a random double-blind study. Sci Total Environ, 2022, 851: 157966. |
| [39] |
Liu J, Ai Y, Niu X, et al. Taurine protects against cardiac dysfunction induced by pressure overload through SIRT1-p53 activation. Chem Biol Interact, 2020, 317: 108972. |
| [40] |
Yang YJ, Han YY, Chen K, et al. TonEBP modulates the protective effect of taurine in ischemia-induced cytotoxicity in cardiomyocytes. Cell Death Dis, 2015, 6: e2025. |
| [41] |
Guizoni DM, Freitas IN, Victorio JA, et al. Taurine treatment reverses protein malnutrition-induced endothelial dysfunction of the pancreatic vasculature: the role of hydrogen sulfide. Metabolism, 2021, 116: 154701. |
| [42] |
Hussy N, Deleuze C, Desarménien MG, et al. Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol, 2000, 62: 113-34. |
| [43] |
Hussy N, Deleuze C, Pantaloni A, et al. Agonist action of taurine on glycine receptors in rat supraoptic magnocellular neurones: possible role in osmoregulation. J Physiol, 1997, 502: 609-21. |
| [44] |
赵丽芳, 李芳, 刘敬, 等. 牛磺酸及其对中枢神经系统的保护作用. 中华实用儿科临床杂志, 2015, 30: 635-7. |
| [45] |
Huxtable R, Bressler R. Effect of taurine on a muscle intracellular membrane. Biochim Biophys Acta, 1973, 323: 573-83. |
| [46] |
Schaffer SW, Jong CJ, Ramila KC, et al. Physiological roles of taurine in heart and muscle. J Biomed Sci, 2010, 17(Suppl 1): S2. |
| [47] |
许思毛, 刘涛波, 苏全生. 大负荷运动对大鼠心肌细胞膜钠钾泵, 钙泵与肌浆网钙泵活性的影响. 体育科学, 2010, 30: 82-6. |
| [48] |
Jang HJ, Kim SJ. Taurine exerts anti-osteoclastogenesis activity via inhibiting ROS generation, JNK phosphorylation and COX-2 expression in RAW264.7 cells. J Recept Signal Transduct Res, 2013, 33: 387-91. |
| [49] |
Tang Y, Kim YS, Choi EJ, et al. Taurine attenuates epithelial-mesenchymal transition-related genes in human prostate cancer cells. Adv Exp Med Biol, 2017, 975pt2: 1203-12. |
| [50] |
Wang G, Ma N, He F, et al. Taurine attenuates carcinogenicity in ulcerative colitis‐colorectal cancer mouse model. Oxid Med Cell Longev, 2020, 2020: 7935917. |
| [51] |
Niu Y, Ma F, Huang W, et al. Long non-coding RNA TUG1 is involved in cell growth and chemoresistance of small cell lung cancer by regulating LIMK2b via EZH2. Mol Cancer, 2017, 16: 5. |
| [52] |
Taurine market size. Verified Market Reports, 2025[R/OL]. https://www.verifiedmarketreports.com/zh/product/taurine-market/
|
| [53] |
Bulychev EY, Rubanyak NY. Commercial synthesis of 2-aminoethanesulfonic acid (taurine). Pharm Chem J, 2013, 46: 740-1. |
| [54] |
Yamamoto S. Plasma taurine in liver cirrhosis with painful muscle cramps. Adv Exp Med Biol, 1996, 403: 597-600. |
| [55] |
Trautwein EA, Hayes KC. Taurine concentrations in plasma and whole blood in humans: estimation of error from intra-and interindividual variation and sampling technique. Am J Clin Nutr, 1990, 52: 758-64. |
| [56] |
Agnello G, Chang LL, Lamb CM, et al. Discovery of a substrate selectivity motif in amino acid decarboxylases unveils a taurine biosynthesis pathway in prokaryotes. ACS Chem Biol, 2013, 8: 2264-71. |
| [57] |
Tevatia R, Allen J, Rudrappa D, et al. The taurine biosynthetic pathway of microalgae. Algal Res, 2015, 9: 921-6. |
| [58] |
Miyazaki T. Identification of a novel enzyme and the regulation of key enzymes in mammalian taurine synthesis. J Pharmacol Sci, 2024, 154: 9-17. |
| [59] |
Veeravalli S, Phillips IR, Freire RT, et al. Flavin-containing monooxygenase 1 catalyzes the production of taurine from hypotaurine. Drug Metab Dispos, 2020, 48: 378-85. |
| [60] |
Cavallini D, De Marco C, Mondovi B, et al. The biological oxidation of hypotaurine. Biochim Biophys Acta, 1954, 15: 301-3. |
| [61] |
Liu P, Ge X, Ding H, et al. Role of glutamate decarboxylase-like protein 1(GADL1) in taurine biosynthesis. J Biol Chem, 2012, 287: 40898-906. |
| [62] |
孙林娟, 陈彪. 半胱胺的药理作用及应用研究. 中国实用医药, 2010, 5: 235-7. |
| [63] |
Berruyer C, Pouyet L, Millet V, et al. Vanin-1 licenses inflammatory mediator production by gut epithelial cells and controls colitis by antagonizing peroxisome proliferator-activated receptor γ activity. J Exp Med, 2006, 203: 2817-27. |
| [64] |
Driggers CM, Hartman SJ, Karplus PA. Structures of Arg-and Gln-type bacterial cysteine dioxygenase homologs. Protein Sci, 2015, 24: 154-61. |
| [65] |
邓洁, 吴巧芬, 高华, 等. 一个新的L-半胱亚磺酸脱羧酶基因的分析和突变. 微生物学报, 2017, 57: 1283-92. |
| [66] |
Jiang C, Wu B. Molecular cloning and functional characterization of a novel decarboxylase from uncultured microorganisms. Biochem Biophys Res Commun, 2007, 357: 421-6. |
| [67] |
Deng J, Wu Q, Gao H, et al. Molecular characterization and directed evolution of a metagenome-derived L-cysteine sulfinate decarboxylase. Food Technol Biotechnol, 2018, 56: 117. |
| [68] |
Joo YC, Ko YJ, You SK, et al. Creating a new pathway in Corynebacterium glutamicum for the production of taurine as a food additive. J Agric Food Chem, 2018, 66: 13454-63. |
| [69] |
Tevatia R, Payne S, Allen J, et al. A synthetic cdo/csad taurine pathway in the green unicellular alga Chlamydomonas reinhardtii. Algal Res, 2019, 40: 101491. |
| [70] |
Xuan W, Gen H, Kangsen M, et al. Differential regulation of taurine biosynthesis in rainbow trout and Japanese flounder. Sci Rep, 2016, 6: 21231. |
| [71] |
Wei W, Lyu X, Markhard LA, et al. PTER is a N-acetyltaurine hydrolase that regulates feeding and obesity. Nature, 2024, 633: 182-8. |
| [72] |
Wei Y, Zhang Y. Glycyl radical enzymes and sulfonate metabolism in the microbiome. Annu Rev Biochem, 2021, 90: 817-46. |
| [73] |
Kondo H, Ishimoto M. Taurine dehydrogenase. Methods Enzymol, 1987, 143: 496-9. |
| [74] |
Kondo H, Anada H, Osawa K, et al. Formation of sulfoacetaldehyde from taurine in bacterial extracts. J Biochem, 1971, 69: 621-3. |
| [75] |
Denger K, Ruff J, Schleheck D, et al. Rhodococcus opacus expresses the xsc gene to utilize taurine as a carbon source or as a nitrogen source but not as a sulfur source. Microbiology, 2004, 150: 1859-67. |
| [76] |
Fernandez ME, Bernier M, Price NL. Is taurine an aging biomarker?. Science, 2025, 388: eadl2116. |
 2025, Vol. 37
2025, Vol. 37 

 叶海峰,华东师范大学特聘教授、博士生导师、生命科学学院院长、国家重点研发计划首席科学家、国家万人计划领军人才、中青年科技创新领军人才、优青、青千、上海市首批尚思探索学者。主持国家自然科学基金原创探索项目、重点项目,以及上海市合成生物重点专项等。任教育部科技委交叉科学与未来技术专门委员会委员、中国生物工程学会理事。2007–2013年于瑞士苏黎世联邦理工学院(ETH Zurich)从事博士和博士后研究工作。2013年被授予ETH Zurich最高荣誉奖章。叶海峰博士于2014年回到华东师范大学生命科学学院工作,组建医学合成生物学实验室。课题组聚焦人工生物分子机器的设计与动态调控研究,致力于构建具有创新机制的遗传控制系统,并以此为基础开发智能化活体药物体系。研究方向重点围绕代谢性疾病、肿瘤及衰老等重大健康问题,旨在通过多学科交叉融合,探索精准治疗的全新策略与技术路径,为相关疾病的临床干预提供理论支撑与应用范式。近年来,基于合成生物学理念,建立了一系列控制遗传技术平台,开发了智能活体药物用于代谢病和肿瘤的精准可控的基因治疗和细胞治疗,相关研究成果以通讯作者身份发表在Sci Transl Med、Sci Adv、Nat Biotech、Nat Cancer、Nat Biomed Eng、Nat Chem Biol、Nat Commun、Proc Natl Acad Sci USA、Mol Cell、Cell Syst、Cell Rep Med等期刊。申请发明专利20余项,授权10余项;
叶海峰,华东师范大学特聘教授、博士生导师、生命科学学院院长、国家重点研发计划首席科学家、国家万人计划领军人才、中青年科技创新领军人才、优青、青千、上海市首批尚思探索学者。主持国家自然科学基金原创探索项目、重点项目,以及上海市合成生物重点专项等。任教育部科技委交叉科学与未来技术专门委员会委员、中国生物工程学会理事。2007–2013年于瑞士苏黎世联邦理工学院(ETH Zurich)从事博士和博士后研究工作。2013年被授予ETH Zurich最高荣誉奖章。叶海峰博士于2014年回到华东师范大学生命科学学院工作,组建医学合成生物学实验室。课题组聚焦人工生物分子机器的设计与动态调控研究,致力于构建具有创新机制的遗传控制系统,并以此为基础开发智能化活体药物体系。研究方向重点围绕代谢性疾病、肿瘤及衰老等重大健康问题,旨在通过多学科交叉融合,探索精准治疗的全新策略与技术路径,为相关疾病的临床干预提供理论支撑与应用范式。近年来,基于合成生物学理念,建立了一系列控制遗传技术平台,开发了智能活体药物用于代谢病和肿瘤的精准可控的基因治疗和细胞治疗,相关研究成果以通讯作者身份发表在Sci Transl Med、Sci Adv、Nat Biotech、Nat Cancer、Nat Biomed Eng、Nat Chem Biol、Nat Commun、Proc Natl Acad Sci USA、Mol Cell、Cell Syst、Cell Rep Med等期刊。申请发明专利20余项,授权10余项; 田进忠,华东师范大学生命科学学院生物医学系研究员、博士生导师。主要从事医学合成生物学与抗衰老相关领域研究。在Nature Metabolism、Nature Communications、Nucleic Acids Research、Synthetic and Systems Biotechnology、Applied Microbiology and Biotechnology等国际知名学术期刊发表SCI论文10余篇。申请国内外专利5项。承担国家、省部级科研项目4项。兼任中国生物工程学会青年委员,上海市生物工程学会合成生物学专业委员会委员。先后担任Nature Communications、The Innovation、ACS Synthetic Biology、Applied Microbiology and Biotechnology等学术期刊审稿人
田进忠,华东师范大学生命科学学院生物医学系研究员、博士生导师。主要从事医学合成生物学与抗衰老相关领域研究。在Nature Metabolism、Nature Communications、Nucleic Acids Research、Synthetic and Systems Biotechnology、Applied Microbiology and Biotechnology等国际知名学术期刊发表SCI论文10余篇。申请国内外专利5项。承担国家、省部级科研项目4项。兼任中国生物工程学会青年委员,上海市生物工程学会合成生物学专业委员会委员。先后担任Nature Communications、The Innovation、ACS Synthetic Biology、Applied Microbiology and Biotechnology等学术期刊审稿人