合成生物学作为一个融合了生物学、工程学等多学科的交叉领域,旨在利用生物体内的模块化元件构建系统性基因回路,实现在特定环境信号输入下生物体的反馈与输出[1]。历经长期的探索与积累,研究者对多种生物系统进行精准重编程来实现特定的功能,使合成生物学在生物制造、环境监测、可再生能源开发以及生物安全等多个领域展现出广泛应用前景[2-4]。
随着临床需求的不断扩大,将合成生物学中模块化调控理念合理引入活体药物的构建具有重要意义[5]。该策略不仅拓展了合成生物学的应用边界,更催生了“医学合成生物学”这一新兴理念,为临床疾病治疗提供了全新的思路[6-8]。
近年来,依托医学合成生物学理念的研究在细胞免疫治疗、基因治疗等多个领域取得了重要进展[9, 10]。在细胞免疫治疗方面,最具代表性的是嵌合抗原受体T细胞(chimeric antigen receptor T cell, CAR-T)疗法,其通过识别特定肿瘤抗原并激活T细胞下游信号转导通路实现肿瘤杀伤[11-13]。同时,为提高治疗安全性,研究者进一步引入微环境信号响应[14, 15]、逻辑门[16-19]和活性诱导型CAR[20-22]等调控机制,以降低其脱靶效应和系统毒性[23]。除CAR-T疗法外,基于自然杀伤细胞(natural killer cell, NK)和巨噬细胞(macrophage)构建的CAR-NK和CAR-M疗法也展现出良好的抗肿瘤潜力[24, 25]。在基因治疗领域,研究者构建了肿瘤微环境响应的基因递送载体[26-27],实现了治疗基因在肿瘤组织中的高效、特异性递送。除上述方向外,在合成生物学思路的指导下,研究者也开发了一系列可响应疾病微环境或外源刺激信号(如磁场、超声等)的前体药物,旨在进一步降低脱靶效应,提高治疗精度[28-31]。
然而,尽管免疫细胞疗法(如CAR-T)在血液系统恶性肿瘤中已取得显著临床成效,其在实体瘤治疗中的应用仍面临诸多挑战。实体瘤特有的免疫抑制微环境显著限制了CAR-T细胞的浸润、存活和功能。此外,部分肿瘤相关抗原在正常组织中的低水平表达显著增加了CAR-T细胞的脱靶风险,进一步影响了其在实体瘤治疗中的安全性。因此,开发靶向性更强且能克服肿瘤微环境限制的新一代治疗策略已成为当前肿瘤免疫治疗领域的重要研究方向。
在合成生物学已在多种治疗方向展现出重要应用价值的基础上,近年来,溶瘤细菌疗法与溶瘤病毒疗法作为新兴的癌症治疗手段也逐渐受到关注,通过理性设计与改造使细菌和病毒成为抗肿瘤利器并解决上述疗法面临的难题则有望为临床提供全新的治疗范式[32]。鉴于此,本文将聚焦于合成生物学在指导细菌治疗与溶瘤病毒构建中的研究进展,以期进一步阐明其在新型活体药物开发中的潜在价值与应用前景。
1 合成生物学在细菌疗法中的应用 1.1 细菌治疗概述细菌疗法可追溯至19世纪末,William B. Coley观察到一位多次复发的颈部软组织肉瘤患者在感染链球菌后,其肿瘤出现了明显的消退。这一发现促使他开发出一种由加热灭活的链球菌(Streptococcus pyogenes)和粘质沙雷氏菌(Serratia marcescens)混合而成的细菌制剂,即“Coley毒素”。经过多轮临床实践,“Coley毒素”在多个肿瘤类型中均取得一定疗效,其中部分软组织肉瘤和淋巴肉瘤患者实现了长期缓解,甚至治愈[33]。然而,受限于当时对免疫机理的认知,加之该疗法存在较大的毒副作用以及放射治疗等手段已然在临床应用上取得巨大成效,细菌疗法逐渐被边缘化。时至今日,伴随免疫治疗研究的逐渐深入以及合成生物学和基因工程技术的迅猛发展,细菌治疗重新受到研究者的青睐。
相较于传统肿瘤治疗手段,细菌治疗具有如下优势:(1)通过对肿瘤免疫抑制微环境的趋化作用,细菌能主动定植于原发性及转移性肿瘤组织,实现肿瘤特异性靶向[34, 35];(2)作为药物递送载体,可实现多种治疗分子的原位表达与释放,即在显著降低系统毒性的同时,提高药物局部浓度从而增强抗肿瘤效应[36-38];(3)细菌不仅可通过自身毒性因子直接杀伤肿瘤细胞,还可借助其组分(如鞭毛蛋白、脂多糖等)以及由肿瘤细胞裂解释放的肿瘤相关性和肿瘤特异性抗原来激活宿主免疫系统,进而诱导强效的抗肿瘤免疫应答[39-41];(4)此外,细菌治疗具备良好的可控性,通过引入环境响应元件、自杀开关等合成生物学工具,可实现对治疗活性的精准时空调控[42, 43]。
然而,尽管细菌疗法在临床前研究中展现出相当大的潜力,但至今仍未有获批上市的相关药物,其中主要存在安全性与有效性这两方面问题:(1)在安全性方面,由于细菌本身为病原微生物,其在体内正常组织中的非特异性分布以及细菌部分组分(如内毒素等)可引发严重的系统免疫炎症反应[44];(2)在有效性方面,以该疗法代表性菌株减毒沙门氏菌VNP20009为例,已有Ⅰ期临床研究指出,在安全剂量范围内,VNP20009在患者肿瘤部位定植数量较少,且并未产生显著抗肿瘤效应[45]。
1.2 合成生物学策略指导设计细菌治疗在上述背景下,引入合成生物学策略无疑可为细菌治疗面临的难题提供新的解决思路。通过构建可响应肿瘤微环境(tumor micro-environment, TME)的基因调控回路,有望提升细菌对肿瘤组织的靶向能力,并降低其在正常组织的分布[46];此外,借助模块化设计,细菌可搭载表达毒素、治疗性蛋白和免疫激动剂等药物负载单元,或与免疫治疗、细胞治疗等策略联合使用,以增强其抗肿瘤活性[47]。以下将从提高治疗安全性与有效性这两个层面,阐述合成生物学如何系统性地指导溶瘤细菌的理性设计与功能优化。
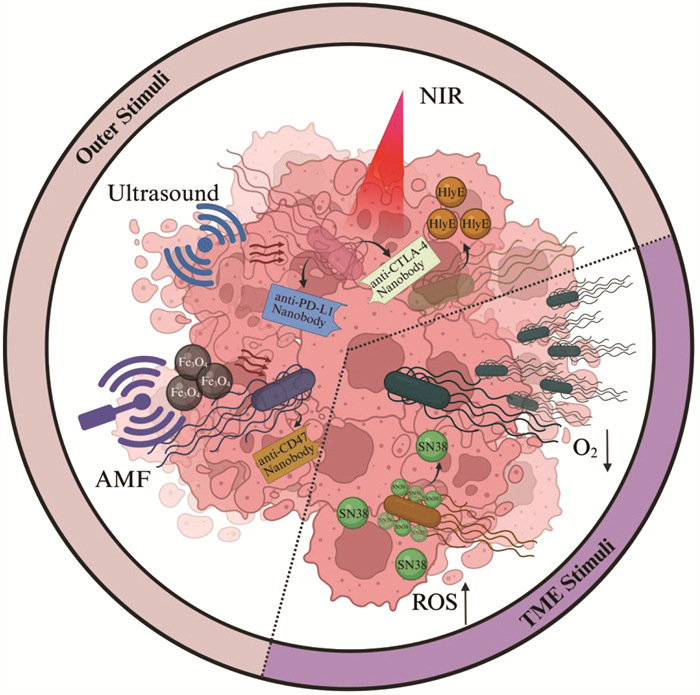
1.2.1 安全性在提高安全性层面,大体可分为构建基因调控回路以响应TME或外源信号刺激(outer stimuli)这两类细菌治疗体系(图 1)。
|
本文提及的外源信号刺激包括近红外光(NIR)、超声以及交变磁场(AMF)等;肿瘤微环境刺激包括乏氧、活性氧等。Created in BioRender. Yuan, J. (2025) https://BioRender.com/hm0ckvd 图 1 利用合成生物学策略设计响应TME和外源信号刺激的溶瘤细菌疗法 |
基于肿瘤的乏氧微环境[48],刘陈立团队[49]通过将沙门氏菌中合成细胞壁肽聚糖前体二氢二氨基庚二酸的关键基因asd置于乏氧响应启动子元件调控下,实现了携带有完整脂多糖的沙门氏菌在肿瘤部位的特异性定植,并展现出显著的抗肿瘤效应。同时,该研究首次揭示了该工程菌株利用白细胞介素-10 (interleukin-10, IL-10)受体的滞后表达机制同时实现细菌免疫逃逸和肿瘤杀伤的关键机理。除乏氧外,TME中存在广泛的氧化还原失衡,表现为抗氧化能力下降、活性氧(reactive oxygen species, ROS)异常累积以及氧化应激相关信号通路失调[50]。基于此,Chang团队[51]在筛选出的具有高效鼻咽癌靶向的胚芽乳杆菌WFCS1治疗载体中构建了利用氧化还原响应型连接子偶联拓扑异构酶I抑制剂SN38的前体药物递送系统,极大降低了该药物的系统毒性并显著提升了抗肿瘤活性。此外,基于TME中乳酸积累、乏氧等特征,Danino团队[46]系统性结合了环境响应元件和逻辑门中“与门”的理念开发了多种生物传感器,极大提高了细菌在肿瘤和肝脏中的定植比例,进一步实现肿瘤富集。
1.2.1.2 响应外源信号刺激的溶瘤细菌疗法在响应外源信号刺激方面,研究者围绕光、磁、热、超声等外源调控模式进行了广泛研究[52]。最近,叶海峰团队[53]开发了近红外(near-infrared, NIR)响应的光敏色素嵌合调控系统,即在近红外光辐照下细菌可于肿瘤原位分泌靶向程序性死亡配体-1 (programmed death-ligand 1, PD-L1)和细胞毒性T淋巴细胞相关抗原-4 (cytotoxic T-lymphocyte-associated antigen 4, CTLA-4)的纳米抗体(nanobody),并在多种肿瘤模型中验证了杀伤效果。类似地,金帆团队[54]构建了基于减毒铜绿假单胞菌的近红外调控体系,实现了对于细菌生物膜形成与裂解释放细胞毒素溶血素E (hemolysin E, HlyE)的精确时空调控。此外,聂广军团队[55]基于交变磁场(alternating magnetic field, AMF)调控体系,构建了一套顺磁性Fe3O4纳米材料修饰的溶瘤细菌,即在细菌通过表面展示的硫酸乙酰肝素蛋白聚糖(一种肿瘤高表达抗原)的结合配体靶向肿瘤组织后,由纳米材料介导产生磁热转换,从而激活细菌内的热响应转录元件,使细菌原位裂解并表达针对肿瘤表面分化簇47 (cluster of differentiation 47, CD47)分子的nanobody,以此实现肿瘤杀伤。在超声响应方面,Shapiro团队[56]通过超声(ultrasound)调控热敏感抑制因子Tcl42 (λ噬菌体cI蛋白的一种突变体)的降解,实现下游Bxb1整合酶(源自噬菌体的一种丝氨酸整合酶)的表达,其进一步引导原本倒置的P7启动子序列翻转并恢复转录活性,从而开启下游靶向PD-L1和CTLA-4的nanobody的表达。
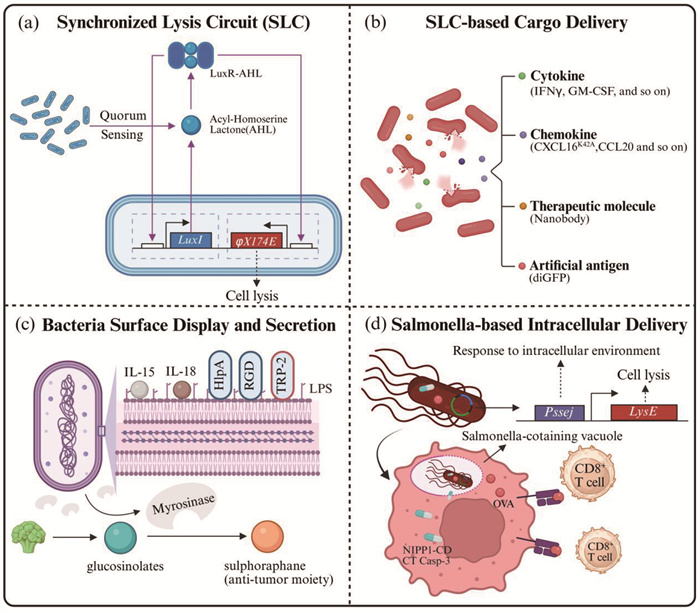
1.2.2 有效性在提高有效性层面,研究者通过基因工程改造,将溶瘤细菌进一步改造成药物或抗原递送载体,将目的蛋白通过同步裂解基因回路、胞膜展示和胞外分泌等形式递送至肿瘤细胞微环境或细胞内,实现更有效的肿瘤杀伤(图 2)。
|
a,细菌浓度响应的同步裂解基因回路(SLC),其中LuxI为编码酰基高丝氨酸内酯(AHL)合成酶的基因,LuxR为AHL响应元件,与AHL结合后可驱动启动子下游基因如细菌裂解蛋白φX174E的表达。b,基于SLC的货物递送系统,货物类型包括各式细胞因子、趋化因子、治疗性分子以及相关抗原。c,开发细菌表面分子展示平台以及分泌系统以增强细菌疗法疗效与肿瘤靶向性。d,基于沙门氏菌胞内入侵特性的肿瘤细胞胞内治疗性药物以及抗原的递送策略。Created in BioRender. Yuan, J. (2025) https://BioRender.com/mwugtoq 图 2 利用合成生物学策略提高细菌疗法的有效性 |
基于Bhatia和Hasty团队[57]构建的细菌浓度响应的同步裂解基因回路(synchronized lysis circuit, SLC)(图 2a),研究者开发了一系列在TME中持续性地释放细胞因子、趋化因子、治疗性蛋白和人工抗原等的治疗策略(图 2b)。Arpaia团队[58]将该体系引入益生菌E. coli Nissle 1917 (以下简称EcN),于肿瘤原位释放干扰素γ (interferon γ, IFNγ)从而激活肿瘤抗原特异性免疫,并通过激活具有细胞杀伤功能的Foxp3- CD4+和CD8+ T淋巴细胞来打破PD-L1阻断治疗耐受。除细胞因子外,该团队[59]还利用SLC表达人源C-X-C基序趋化因子配体16的K42A突变体(C-X-C motif chemokine ligand 16 K42A mutant, CXCL16K42A)和C-C基序趋化因子配体20 (C-C motif chemokine ligand 20, CCL20),通过招募树突状细胞和CD8+ T淋巴细胞同时激活固有免疫和适应性免疫,实现肿瘤杀伤。类似地,Danino团队[60]通过进一步优化SLC体系,实现细菌于肿瘤原位高效分泌靶向PD-L1和CTLA-4的纳米抗体和粒细胞-巨噬细胞集落刺激因子(granulocyte- macrophage colony-stimulating factor, GM-CSF),在免疫抑制的CT26结直肠癌模型中取得显著疗效。在细菌治疗与其他疗法联合应用方面,该团队[61]在EcN中利用SLC递送能锚定于TME中胶原蛋白和纤维连接蛋白的人工抗原diGFP,同时结合针对该肿瘤抗原的CAR-T疗法,在三阴性乳腺癌模型中证明了该策略的有效性。
1.2.2.2 基于细菌包膜展示和分泌的递送策略除了利用SLC进行递送,研究者通过基因工程化改造,开发了一系列细菌膜表面展示以及胞外分泌的递送平台(图 2c)。在膜展示方面,已有研究成功展示了IL-15[62]、IL-18[62]、RGD-4C (一种靶向整合素的环肽)[63]、酪氨酸酶相关蛋白-2 (tyrosinase related protein-2, TRP-2)[64]等分子。在胞外分泌方面,基于细菌自然进化的多型分泌体系[65],研究者也已实现对于多种细胞毒素[66-68]、细胞因子[69-71]、纳米抗体[53]等的分泌,实现了对肿瘤细胞的直接或间接杀伤。更值得一提的是,Chang团队[72]将上述两种策略有机结合,于大肠杆菌表面展示了组蛋白样蛋白A (histone-like protein A, HlpA),增强了细菌对于结直肠癌细胞的靶向;同时,通过分泌表达黑芥子酶(myrosinase),将通过饮食摄入的葡萄糖异硫氰酸酯(glucosinolates)转化为具有抗肿瘤活性的异硫氰酸酯类化合物(如萝卜硫素,sulphoraphane),二者协同作用实现了兼顾安全性与有效性的肿瘤治疗模式。
1.2.2.3 基于胞内入侵与逃逸特性的递送策略值得注意的是,上述提及的细菌递送策略是将货物分子通过展示或分泌的策略递送到TME中并于肿瘤细胞外发挥作用。与之相对,基于沙门氏菌细胞内入侵的特性,Forbes团队[73, 74]通过构建沙门氏菌含泡响应裂解的基因回路Pssej-LysE,开发了一套基于VNP20009的蛋白胞内递送体系,先后实现了对NIPP1-CD (核抑制剂NIPP1的C端结构域)、CT Casp-3 (肿瘤靶向性半胱天冬酶-3)以及抗原卵清白蛋白(ovalbumin, OVA)的递送(图 2d)。此外,Arpaia和Danino团队[75]在EcN中引入可使溶酶体膜通透化的成孔蛋白李斯特菌素O,使细菌在被巨噬细胞吞噬后完成胞内逃逸并递送肿瘤新抗原,以此发展了一个能够治疗晚期实体瘤的肿瘤疫苗平台。
1.3 细菌治疗临床进展综上所述,利用合成生物学策略对细菌底盘进行改造,极大推动了细菌治疗的科学研究进程与临床应用潜力。表 1汇总了部分进入临床Ⅰ/Ⅱ期和临床Ⅱ期的细菌疗法。值得注意的是,目前已进入临床阶段的溶瘤细菌疗法多采用联合用药策略,体现出其在单药疗效、免疫激活等方面仍存在一定局限性,而与免疫检查点抑制剂、小分子药物或癌症疫苗联合用药则可进一步增强抗肿瘤免疫应答、改善肿瘤微环境,并最终实现有效抑制肿瘤进展。整体来看,现有临床方案在安全性控制、靶向性提升及联合协同机制上不断优化,显示出“工程改造+ 联合治疗”的发展趋势。
| 表 1 部分进入临床Ⅰ/Ⅱ期和临床Ⅱ期的细菌疗法 |
溶瘤病毒疗法起源于20世纪初,1904年《柳叶刀》杂志首次记录了流感病毒感染引发白血病患者外周血白细胞计数显著下降的病例[83],揭示了病毒与肿瘤消退之间的潜在关联。1912年,意大利病理学家De Pace[84]报道了一例宫颈癌患者在接种减毒狂犬疫苗后原发病灶体积缩小的现象,进一步强化了病毒可用于“以毒攻瘤”的设想。这些临床现象推动了早期对溶瘤病毒的探索,一批野生型病毒如肝炎病毒、黄热病病毒等受到研究者关注[85]。
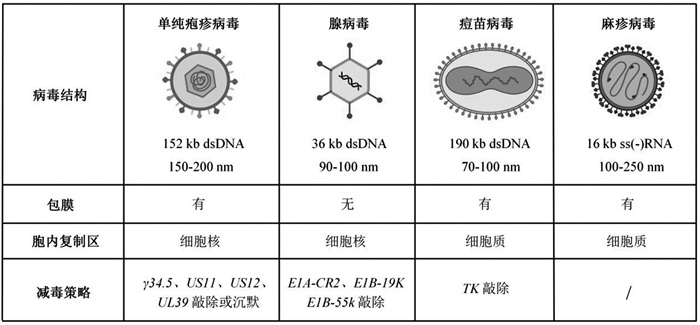
随着基因工程与合成生物学技术的发展,研究者得以对病毒进行结构优化与功能重构,从而提升其肿瘤靶向性与治疗安全性。截至目前,临床研究涵盖了包括单纯疱疹病毒(herpes simplex virus, HSV)、腺病毒(adenovirus, Ad)、痘苗病毒(vaccinia virus, VV)及麻疹病毒(measles virus, MV)在内的多种基因工程化载体(表 2)。下面将围绕上述病毒载体来阐述合成生物学在溶瘤病毒底盘设计中的指导作用。
| 表 2 临床上发展的常用溶瘤病毒载体 |
1型单纯疱疹病毒是在免疫治疗中研究最广泛的双链DNA病毒之一,基因组大小超过150 kb,为转基因改造提供了充足的空间;同时,其基因组由UL和US片段组成,这种结构利于转基因片段的插入以及病毒内部毒性相关基因的删除,以此获得更优的HSV-1突变体[86]。
在沉默毒性基因以提高安全性方面,研究者们先后探索了沉默HSV基因组结构中的γ34.5 (ICP34.5)[87, 88]、US11[89]、US12 (ICP47)[90]、UL39 (ICP6)[91]等,以期通过限制病毒的复制和组装、减少病毒免疫逃逸等方式来降低HSV对于正常细胞的毒性。研究人员将外源性ICP4插入ICP4基因敲除的HSV-1病毒中,并使其受到肿瘤特异性启动子(如缺氧诱导因子反应性启动子,白蛋白、钙调蛋白和癌胚抗原启动子等)调控,以提高病毒的肿瘤特异性[92-96]。
此外,为增强溶瘤病毒的治疗效果,针对HSV-1基因组插入改造的研究更为广泛。Chiocca团队[97]和刘仁斌团队[98]分别在病毒基因组中引入了促凋亡基因GADD34和MyD116以替代γ34.5,增强病毒对于肿瘤细胞的选择性杀伤。梁廷波团队[99, 100]利用编码IL-12、IL-15、IL-15RA1 (IL-15受体α亚基异构体1型)和免疫检查点PD-L1阻断肽的HSV实现了更强的肿瘤杀伤效果。除细胞因子外,为增强免疫细胞在肿瘤部位的浸润,部分趋化因子也被纳入到HSV的基因组结构中[101, 102]。Yu团队[103]利用病毒表达西妥昔单抗的单链可变区片段抗体(single-chain variable fragment, scFv)与CCL5的异源二聚体,显著增强了自然杀伤细胞、巨噬细胞和T淋巴细胞的迁移和活化,并于一定程度上延长了小鼠的生存期。此外,将免疫检查点抑制剂基因引入病毒编码区,可以利用二者的协同作用逆转杀伤型T淋巴细胞耗竭以实现肿瘤杀伤[104-117]。除表达上述治疗性蛋白外,Wakimoto团队[108]构建了一种能够表达光敏蛋白KillerRed的溶瘤单纯疱疹病毒G47Δ-KR,其在激光照射后产生的ROS可实现对神经恶性肿瘤的有效杀伤。
2.2.2 腺病毒腺病毒是一种无包膜的基因组大小约为36 kb的双链DNA病毒[109]。其优势在于:(1)病毒基因组体内非整合的特性确保了临床用药的稳定性和生物安全性[110];(2)利用同源重组技术能够对腺病毒实现精准的基因编辑[111];(3)在工业化制备层面已实现了高滴度生产(>1012 VP/mL)[112]。
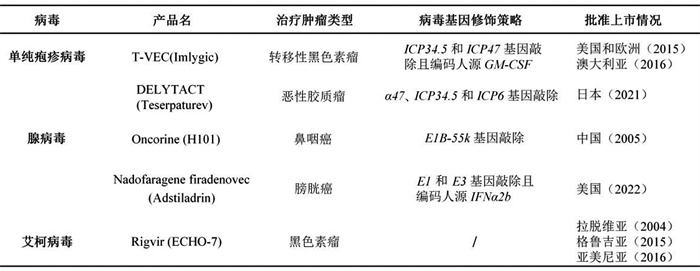
基于Ad5在细胞内借助E1B-55K基因编码产物与TP53蛋白互作以阻断细胞凋亡信号转导并实现病毒复制的自然机理[113, 114],研究者通过定向敲除E1B-55K基因,成功研制了具有肿瘤选择性复制特征的重组人5型腺病毒注射液H101[115]。作为全球首个获得国家药品监管部门批准上市的基因工程溶瘤病毒药物,H101标志着我国在溶瘤病毒治疗领域取得了重要研究进展[116]。
值得注意的是,基于E1B-55K基因单一位点改造的溶瘤腺病毒仅能在TP53信号通路异常的肿瘤类型中实现选择性复制,这无疑限制了其临床应用范围。为此,研究者通过将肿瘤组织特异性启动子置于病毒必需基因的调控区域,进一步提高了溶瘤病毒的肿瘤嗜性。基于端粒酶在多数肿瘤中异常激活的现象[117],Fujiwara团队[118-120]构建了由人源端粒酶逆转录酶启动子调控表达病毒复制必需基因的病毒载体,其显著降低了对正常肝细胞的毒性并极大提高了病毒在肝癌HepG2细胞、胰腺癌PANC-1细胞及胃癌MKN45细胞中的复制效率。除此之外,基于肿瘤广谱性或特异性抗原,如生存素[121]、环氧合酶-2[122]、甲胎蛋白[123]和癌胚抗原[124]等的启动子,也常用于溶瘤腺病毒的设计。同理,谢震团队[125]构建了包含可编程模块化合成基因线路的腺病毒载体,即该病毒能通过响应特异性启动子和环境中的microRNA实现在肝癌细胞中选择性复制并释放免疫效应物,由此提高了该疗法对肿瘤组织的靶向能力和杀伤效果。
2.2.3 痘苗病毒痘苗病毒是一种有包膜的基因组约190 kb的双链DNA病毒,结构复杂且不对称,由被单个脂蛋白膜包裹的核蛋白核心组成。其中,胸苷激酶(thymidine kinase, TK)基因编码病毒复制必需基因,且该基因通常在恶性细胞中高表达而在正常细胞中表达量低,故早期研究者将病毒基因组中的胸苷激酶基因删除,获得了在肿瘤细胞中特异性复制的病毒改造株[126]。在此基础上,为增强痘苗病毒的抗肿瘤效果,研究者于其基因组上引入一系列编码细胞因子的基因,如IL-12[127]、IL-15[128]、IL-36γ[129]、IL-21[130]以及募集免疫细胞进入肿瘤病灶的趋化因子CCL5[131]、CXCL11[132]等。
此外,研究表明,痘苗病毒不仅可以通过裂解肿瘤细胞并激活系统免疫将冷肿瘤重塑为热肿瘤,还能通过增加肿瘤细胞表面PD-L1、CTLA-4等的表达,使其对免疫检查点阻断疗法更为敏感[133]。因此,该溶瘤病毒疗法与细胞治疗、免疫治疗等其他疗法的协同策略有望达到更好的临床治疗效果。基于该特性,Chen团队[134]验证了表达PD-L1抑制剂的VV具有良好的治疗效果,并且能够通过引发全身抗肿瘤反应抑制远端肿瘤的生长。魏继武团队[135]则发展了一种表达靶向T细胞免疫球蛋白和ITIM结构域(TIGIT)的scFv的载体VV-scFv-TIGIT,并指出与其他免疫检查点抑制剂联用可产生协同抗肿瘤效应。此外,研究人员也发展了VV与CAR-T疗法的联合应用策略,利用病毒向肿瘤细胞内递送CD19分子作为肿瘤抗原,并联合已获批上市的针对CD19的CAR-T细胞,结果表明可显著抑制肿瘤生长[136, 137],为临床的治疗策略选择指示了新的方向。
2.2.4 麻疹病毒麻疹病毒是一种包膜的基因组长度约16 kb的负链RNA病毒。通过对病毒结构的研究,研究人员将多种不同的靶向单元如scFv[138, 139]、整合素结合肽[140]、DARPin蛋白[141]等插入基因组中血凝素蛋白的C端,以此封闭病毒与受体的结合位点,从而赋予病毒新的靶向性。此外,与前面提及的细菌疗法类似,研究者也构建了一系列响应TME和外界条件刺激的病毒载体。Buchholz团队[142]用基质金属蛋白酶识别的序列替换弗林蛋白酶切割位点,构建了一种于TME中恢复活性的病毒,以提高病毒的肿瘤特异性。Takeda团队[143]将Magnet系统成对的光开关蛋白插入到病毒聚合酶L蛋白的柔性结构域,实现了在蓝光照射下通过蛋白异二聚化开启病毒的复制,构建了一种时空调控MV基因表达和复制的系统。Nettelbeck团队[144]将适体酶融合到MV基因组的UTR区域,能够实现小分子调控的病毒复制与传播。此外,Ungerechts团队[145]在MV的3'UTR区域插入了microRNA-7的靶位点,使得在microRNA-7表达较高的正常细胞中病毒复制被抑制,从而降低病毒对正常组织的毒性。此外,在增强病毒抗肿瘤活性方面,研究者也在病毒基因组上引入了一系列效应因子,如GM-CSF[146]、IL-12[147]、靶向CTLA-4和PD-L1的抗体[148]等,以求达到更优的治疗效果。
2.2.5 其他溶瘤病毒除上述常见溶瘤病毒外,其他病毒如柯萨奇病毒、新城疫病毒等也有较为广泛的研究。Luo团队[149]在柯萨奇病毒基因组中插入多拷贝抑癌miR-145/miR-143靶序列,使其在保留感染和裂解KRAS突变型肺腺癌和TP53/RB1突变型小细胞肺癌细胞能力的同时降低对心肌细胞的毒性。赵永祥团队[150]则开发了一种携带α1, 3GT (α1, 3半乳糖基转移酶基因)的重组新城疫病毒,其可通过诱导超急性排斥反应有效抑制食蟹猴原发性肝细胞癌并延长生存期,并进一步在介入性临床试验中展现出较高的疾病控制率。
2.3 溶瘤病毒递送策略尽管上述各式溶瘤病毒展现出肿瘤治疗前景,但其在临床转化上仍面临诸多挑战。作为外来病原,病毒易被患者体内已存在的中和抗体中和清除,致使肿瘤侵染效率受限;同时其诱发的免疫记忆使得难以实现多次给药,进而影响疗效[151, 152]。因此,发展高效的溶瘤病毒递送策略是当前研究亟需解决的难题。
为解决上述问题,研究者提出了多种基于“活载体”的递送策略。平渊团队[153]利用展示T细胞特异性抗原的细胞膜包裹溶瘤腺病毒,通过抗原与受体相互作用将溶瘤病毒偶联在T细胞表面,实现了溶瘤病毒-T细胞嵌合体的静脉递送,并通过抑制PD-L1基因的表达显著延长小鼠生存期。除T细胞外,间充质干细胞以其低免疫原性与肿瘤趋向性也被广泛应用于溶瘤麻疹病毒[154]、单纯疱疹病毒[155]、腺病毒[156]、痘苗病毒[157]和黏液瘤病毒[158]的递送。除利用正常细胞进行溶瘤病毒递送外,顾臻团队[159]利用液氮速冻处理腺病毒Ad11侵染的小鼠肺癌细胞TC-1,在不破坏病毒后续侵染能力的同时消除了载体TC-1细胞的体内增殖,并以此减少了对于溶瘤病毒的中和与清除。此外,鉴于血脑屏障对溶瘤病毒的通透性限制了其在脑部肿瘤治疗中的组织分布与渗透,Lesniak团队[160, 161]利用美国食品药品监督管理局(FDA)批准的神经干细胞系HB1.F3.CD递送工程化改造的腺病毒,并在Ⅰ期临床试验中表征了相关安全性。
除上述细胞递送策略,材料科学与纳米技术的结合也为病毒递送提供了新的解决方案。顾臻团队[162]最新开发了由胆固醇修饰的DNA和组蛋白通过液液相分离形成的共聚集型囊泡并应用于腺病毒Ad11的递送,在小鼠体内显著提高了溶瘤病毒对于肿瘤组织的侵染水平并抑制了肿瘤的生长。
2.4 溶瘤病毒药物进展截至目前,已有多款溶瘤病毒药物或基于溶瘤病毒载体的药物获批上市(表 3)。
| 表 3 目前全球批准的溶瘤病毒和基于溶瘤病毒载体的药物 |
综上所述,合成生物学模块化设计与精准调控的理念推动发展了多个肿瘤靶向性更强、肿瘤杀伤效果更好的溶瘤细菌和溶瘤病毒治疗策略。然而,尽管上述疗法在诱导肿瘤细胞坏死、重塑肿瘤微环境等方面展现出独特优势,其在临床应用中仍面临诸多挑战。
对于溶瘤细菌疗法,安全性仍是制约其广泛应用的主要因素。在免疫功能低下的癌症晚期患者中,经减毒处理的菌株仍存在引发菌血症或全身性感染的风险。此外,携带治疗基因的工程菌在体内可能发生基因突变、丢失或表达失控,显著影响疗效的一致性与可控性。同时,目前多数溶瘤细菌策略在单独应用时治疗效果有限,常需与免疫检查点抑制剂、放化疗或细胞治疗等联合使用。然而,其协同机制尚未被充分阐明,这无疑限制了联合治疗方案的理性设计和临床拓展。
对于溶瘤病毒疗法,有效性成为其临床应用的关键。首次给药后,宿主迅速对病毒这一强免疫原产生免疫应答,致使在后续治疗中病毒被迅速中和清除,因此难以实现多次给药策略。同时,部分溶瘤病毒在实体瘤中的扩散能力有限,难以有效穿透致密的基质结构及低氧区域。此外,肿瘤局部免疫抑制状态包括免疫细胞浸润不足、免疫抑制因子高表达等均可能削弱溶瘤病毒诱导的抗肿瘤免疫效应。
综上所述,为进一步提升溶瘤细菌与溶瘤病毒疗法的临床转化可行性,未来研究应重点聚焦于以下几个方向:(1)发展更高安全性和稳定性的底盘工程菌与病毒载体;(2)开发更精确且高效的调控表达系统以增强治疗基因的时空特异性表达;(3)设计更高效的靶向递送系统以降低宿主免疫清除并进一步提高药物在肿瘤部位的富集;(4)系统性评估溶瘤细菌与溶瘤病毒疗法与免疫治疗、细胞治疗等现有疗法的联合策略并阐明其协同作用机理。通过对上述方向的深层次研究,有望全面提升治疗的安全性、有效性及临床适用性,为恶性肿瘤患者带来福祉。
| [1] |
Cameron DE, Bashor CJ, Collins JJ. A brief history of synthetic biology. Nat Rev Microbiol, 2014, 12: 381-90. |
| [2] |
Jones EM, Marken JP, Silver PA. Synthetic microbiology in sustainability applications. Nat Rev Microbiol, 2024, 22: 345-59. |
| [3] |
Rabinovitch-Deere CA, Oliver JW, Rodriguez GM, et al. Synthetic biology and metabolic engineering approaches to produce biofuels. Chem Rev, 2013, 113: 4611-32. |
| [4] |
Hoffmann SA, Diggans J, Densmore D, et al. Safety by design: biosafety and biosecurity in the age of synthetic genomics. iScience, 2023, 26: 106165. |
| [5] |
Cubillos-Ruiz A, Guo T, Sokolovska A, et al. Engineering living therapeutics with synthetic biology. Nat Rev Drug Discov, 2021, 20: 941-60. |
| [6] |
Tan X, Letendre JH, Collins JJ, et al. Synthetic biology in the clinic: engineering vaccines, diagnostics, and therapeutics. Cell, 2021, 184: 881-98. |
| [7] |
张强, 顾明亮. 合成生物学的医学应用. 生命的化学, 2021, 41: 113-32. |
| [8] |
崔金明, 王力为, 常志广, 等. 合成生物学的医学应用研究进展. 中国科学院院刊, 2018, 33: 1218-27. |
| [9] |
Zhu B, Yin H, Zhang D, et al. Synthetic biology approaches for improving the specificity and efficacy of cancer immunotherapy. Cell Mol Immunol, 2024, 21: 436-47. |
| [10] |
Zhao N, Song Y, Xie X, et al. Synthetic biology-inspired cell engineering in diagnosis, treatment, and drug development. Signal Transduct Target Ther, 2023, 8: 112. |
| [11] |
Maus MV, June CH. Making better chimeric antigen receptors for adoptive T-cell therapy. Clin Cancer Res, 2016, 22: 1875-84. |
| [12] |
Yip A, Webster RM. The market for chimeric antigen receptor T cell therapies. Nat Rev Drug Discov, 2018, 17: 161-2. |
| [13] |
Clubb JD, Gao TA, Chen YY. Synthetic biology in the engineering of CAR-T and CAR-NK cell therapies: facts and hopes. Clin Cancer Res, 2023, 29: 1390-402. |
| [14] |
Roybal KT, Williams JZ, Morsut L, et al. Engineering T cells with customized therapeutic response programs using synthetic Notch receptors. Cell, 2016, 167: 419-32. |
| [15] |
Zhu X, Chen J, Li W, et al. Hypoxia-responsive CAR-T cells exhibit reduced exhaustion and enhanced efficacy in solid tumors. Cancer Res, 2024, 84: 84-100. |
| [16] |
Roybal KT, Rupp LJ, Morsut L, et al. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell, 2016, 164: 770-9. |
| [17] |
Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med, 2013, 5: 215ra172. |
| [18] |
Tousley AM, Rotiroti MC, Labanieh L, et al. Co-opting signalling molecules enables logic-gated control of CAR T cells. Nature, 2023, 615: 507-16. |
| [19] |
Hamieh M, Mansilla-Soto J, Rivière I, et al. Programming CAR T cell tumor recognition: tuned antigen sensing and logic gating. Cancer Discov, 2023, 13: 829-43. |
| [20] |
Qi J, Tsuji K, Hymel D, et al. Chemically programmable and switchable CAR-T therapy. Angew Chem Int Ed Engl, 2020, 59: 12178-85. |
| [21] |
Wu CY, Roybal KT, Puchner EM, et al. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science, 2015, 350: aab4077. |
| [22] |
Zheng Y, Nandakumar KS, Cheng K. Optimization of CAR-T cell-based therapies using small-molecule-based safety switches. J Med Chem, 2021, 64: 9577-91. |
| [23] |
Flugel CL, Majzner RG, Krenciute G, et al. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat Rev Clin Oncol, 2023, 20: 49-62. |
| [24] |
Dagher OK, Posey AD Jr. Forks in the road for CAR T and CAR NK cell cancer therapies. Nat Immunol, 2023, 24: 1994-2007. |
| [25] |
Li N, Geng S, Dong ZZ, et al. A new era of cancer immunotherapy: combining revolutionary technologies for enhanced CAR-M therapy. Mol Cancer, 2024, 23: 117. |
| [26] |
Liao Z, Hsu S, Tang S, et al. Potential targeting of the tumor microenvironment to improve cancer virotherapy. Pharmacol Ther, 2023, 250: 108521. |
| [27] |
Butler SS, Date K, Okumura T, et al. Membrane-bound MMP-14 protease-activatable adeno-associated viral vectors for gene delivery to pancreatic tumors. Gene Ther, 2022, 29: 138-46. |
| [28] |
Fu X, Hu X. Ultrasound-controlled prodrug activation: emerging strategies in polymer mechanochemistry and sonodynamic therapy. ACS Appl Bio Mater, 2024, 7: 8040-58. |
| [29] |
Xie A, Hanif S, Ouyang J, et al. Stimuli-responsive prodrug-based cancer nanomedicine. EBioMedicine, 2020, 56: 102821. |
| [30] |
Han HH, Wang HM, Jangili P, et al. The design of small-molecule prodrugs and activatable phototherapeutics for cancer therapy. Chem Soc Rev, 2023, 52: 879-920. |
| [31] |
Ngo TL, Wang KL, Pan WY, et al. Immunomodulatory prodrug micelles imitate mild heat effects to reshape tumor microenvironment for enhanced cancer immunotherapy. ACS Nano, 2024, 18: 5632-46. |
| [32] |
Qi Z, Gu J, Qu L, et al. Advancements of engineered live oncolytic biotherapeutics (microbe/virus/cells): preclinical research and clinical progress. J Control Release, 2024, 375: 209-35. |
| [33] |
Starnes CO. Coley's toxins in perspective. Nature, 1992, 357: 11-2. |
| [34] |
Jiang M, Yang Z, Dai J, et al. Intratumor microbiome: selective colonization in the tumor microenvironment and a vital regulator of tumor biology. MedComm, 2023, 4: e376. |
| [35] |
Duong MT, Qin Y, You SH, et al. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp Mol Med, 2019, 51: 1-15. |
| [36] |
Wu L, Bao F, Li L, et al. Bacterially mediated drug delivery and therapeutics: strategies and advancements. Adv Drug Deliv Rev, 2022, 187: 114363. |
| [37] |
Han S, Song Y, Wu X, et al. Advances in bacteria-based drug delivery systems for anti-tumor therapy. Clin Transl Immunol, 2024, 13: e1518. |
| [38] |
Kang SR, Nguyen DH, Yoo SW, et al. Bacteria and bacterial derivatives as delivery carriers for immunotherapy. Adv Drug Deliv Rev, 2022, 181: 114085. |
| [39] |
Chen J, Qiao Y, Chen G, et al. Salmonella flagella confer anti-tumor immunological effect via activating Flagellin/TLR5 signalling within tumor microenvironment. Acta Pharm Sin B, 2021, 11: 3165-77. |
| [40] |
Felgner S, Kocijancic D, Frahm M, et al. Optimizing Salmonella enterica serovar Typhimurium for bacteria-mediated tumor therapy. Gut Microbes, 2016, 7: 171-7. |
| [41] |
Zhang T, Zhang X, Chen J, et al. Harnessing microbial antigens as cancer antigens: a promising avenue for cancer immunotherapy. Front Immunol, 2024, 15: 1411490. |
| [42] |
Feng Z, Wang Y, Xu H, et al. Recent advances in bacterial therapeutics based on sense and response. Acta Pharm Sin B, 2023, 13: 1014-27. |
| [43] |
Xie R, Fan D, Cheng X, et al. Living therapeutics: precision diagnosis and therapy with engineered bacteria. Biomaterials, 2025, 321: 123342. |
| [44] |
Cohen J. The immunopathogenesis of sepsis. Nature, 2002, 420: 885-91. |
| [45] |
Toso JF, Gill VJ, Hwu P, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol, 2002, 20: 142-52. |
| [46] |
Gurbatri CR, Arpaia N, Danino T. Engineering bacteria as interactive cancer therapies. Science, 2022, 378: 858-64. |
| [47] |
Huang X, Pan J, Xu F, et al. Bacteria-based cancer immunotherapy. Adv Sci (Weinh), 2021, 8: 2003572. |
| [48] |
Chen Z, Han F, Du Y, et al. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther, 2023, 8: 70. |
| [49] |
Chang Z, Guo X, Li X, et al. Bacterial immunotherapy leveraging IL-10R hysteresis for both phagocytosis evasion and tumor immunity revitalization. Cell, 2025, 188: 1842-57. |
| [50] |
Glorieux C, Liu S, Trachootham D, et al. Targeting ROS in cancer: rationale and strategies. Nat Rev Drug Discov, 2024, 23: 583-606. |
| [51] |
Shen H, Zhang C, Li S, et al. Prodrug-conjugated tumor-seeking commensals for targeted cancer therapy. Nat Commun, 2024, 15: 4343. |
| [52] |
Kwon SY, Thi-Thu Ngo H, Son J, et al. Exploiting bacteria for cancer immunotherapy. Nat Rev Clin Oncol, 2024, 21: 569-89. |
| [53] |
Qiao L, Niu L, Wang Z, et al. Engineered bacteria for near-infrared light-inducible expression of cancer therapeutics. Nat Cancer, 2025, 6: 612-28. |
| [54] |
Fu S, Zhang R, Gao Y, et al. Programming the lifestyles of engineered bacteria for cancer therapy. Natl Sci Rev, 2023, 10: nwad031. |
| [55] |
Ma X, Liang X, Li Y, et al. Modular-designed engineered bacteria for precision tumor immunotherapy via spatiotemporal manipulation by magnetic field. Nat Commun, 2023, 14: 1606. |
| [56] |
Abedi MH, Yao MS, Mittelstein DR, et al. Ultrasound-controllable engineered bacteria for cancer immunotherapy. Nat Commun, 2022, 13: 1585. |
| [57] |
Din MO, Danino T, Prindle A, et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature, 2016, 536: 81-5. |
| [58] |
Li F, Yang Z, Savage TM, et al. Programmable bacteria synergize with PD-1 blockade to overcome cancer cell-intrinsic immune resistance mechanisms. Sci Immunol, 2024, 9: eadn9879. |
| [59] |
Savage TM, Vincent RL, Rae SS, et al. Chemokines expressed by engineered bacteria recruit and orchestrate antitumor immunity. Sci Adv, 2023, 9: eadc9436. |
| [60] |
Gurbatri CR, Lia I, Vincent R, et al. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci Transl Med, 2020, 12: eaax0876. |
| [61] |
Vincent RL, Gurbatri CR, Li F, et al. Probiotic-guided CAR-T cells for solid tumor targeting. Science, 2023, 382: 211-8. |
| [62] |
Yang S, Sheffer M, Kaplan IE, et al. Non-pathogenic E. coli displaying decoy-resistant IL18 mutein boosts anti-tumor and CAR NK cell responses. Nat Biotechnol, 2024. |
| [63] |
Park SH, Zheng JH, Nguyen VH, et al. RGD peptide cell-surface display enhances the targeting and therapeutic efficacy of attenuated Salmonella-mediated cancer therapy. Theranostics, 2016, 6: 1672-82. |
| [64] |
Kalyanasundram J, Chia SL, Song AA, et al. Surface display of glycosylated tyrosinase-related protein-2 (TRP-2) tumour antigen on Lactococcus lactis. BMC Biotechnol, 2015, 15: 113. |
| [65] |
Green ER, Mecsas J. Bacterial secretion systems: an overview. Microbiol Spectr, 2016, 4: VMBF-0012-2015. |
| [66] |
Nguyen VH, Kim HS, Ha JM, et al. Genetically engineered Salmonella typhimurium as an imageable therapeutic probe for cancer. Cancer Res, 2010, 70: 18-23. |
| [67] |
Kim JS, Park JE, Choi SH, et al. ECM-targeting bacteria enhance chemotherapeutic drug efficacy by lowering IFP in tumor mouse models. J Control Release, 2023, 355: 199-210. |
| [68] |
Loeffler M, Le'Negrate G, Krajewska M, et al. Inhibition of tumor growth using Salmonella expressing Fas ligand. J Natl Cancer Inst, 2008, 100: 1113-6. |
| [69] |
Loeffler M, Le'Negrate G, Krajewska M, et al. Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proc Natl Acad Sci U S A, 2007, 104: 12879-83. |
| [70] |
Yoon W, Park YC, Kim J, et al. Application of genetically engineered Salmonella typhimurium for interferon γ-induced therapy against melanoma. Eur J Cancer, 2017, 70: 48-61. |
| [71] |
Yoon WS, Chae YS, Hong J, et al. Antitumor therapeutic effects of a genetically engineered Salmonella typhimurium harboring TNF-α in mice. Appl Microbiol Biotechnol, 2011, 89: 1807-19. |
| [72] |
Ho CL, Tan HQ, Chua KJ, et al. Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat Biomed Eng, 2018, 2: 27-37. |
| [73] |
Raman V, Van Dessel N, Hall CL, et al. Intracellular delivery of protein drugs with an autonomously lysing bacterial system reduces tumor growth and metastases. Nat Commun, 2021, 12: 6116. |
| [74] |
Raman V, Howell LM, Bloom SMK, et al. Intracellular Salmonella delivery of an exogenous immunization antigen refocuses CD8 T cells against cancer cells, eliminates pancreatic tumors and forms antitumor immunity. Front Immunol, 2023, 14: 1228532. |
| [75] |
Redenti A, Im J, Redenti B, et al. Probiotic neoantigen delivery vectors for precision cancer immunotherapy. Nature, 2024, 635: 453-61. |
| [76] |
Saltzman DA, Heise CP, Hasz DE, et al. Attenuated Salmonella typhimurium containing interleukin-2 decreases MC-38 hepatic metastases: a novel anti-tumor agent. Cancer Biother Radiopharm, 1996, 11: 145-53. |
| [77] |
Zhou S, Lin Y, Zhao Z, et al. Targeted deprivation of methionine with engineered Salmonella leads to oncolysis and suppression of metastasis in broad types of animal tumor models. Cell Rep Med, 2023, 4: 101070. |
| [78] |
Galicia-Carmona T, Arango-Bravo E, Serrano-Olvera JA, et al. ADXS11-001 LM-LLO as specific immunotherapy in cervical cancer. Hum Vaccin Immunother, 2021, 17: 2617-25. |
| [79] |
Manole S, Dumitrescu D, Constantin C, et al. Setting "cold" tumors on fire: cancer therapy with live tumor-targeting bacteria. Med, 2025, 6: 100549. |
| [80] |
Brahmer JR, Drake CG, Wollner I, et al. JNJ-64041757 (JNJ-757), a live, attenuated, double-deleted Listeria monocytogenes-based immunotherapy in patients with NSCLC: results from two phase 1 studies. JTO Clin Res Rep, 2020, 2: 100103. |
| [81] |
Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol, 2015, 33: 1325-33. |
| [82] |
Lauté-Caly DL, Raftis EJ, Cowie P, et al. The flagellin of candidate live biotherapeutic Enterococcus gallinarum MRx0518 is a potent immunostimulant. Sci Rep, 2019, 9: 801. |
| [83] |
Dock G. The influence of complicating diseases upon leukaemia. Am J Med Sci, 1904, 127: 563-92. |
| [84] |
De Pace N. Sulla scomparsa di un enorme cancro vegetante del collo dell'utero senza cura chirurgica. Ginecologia, 1912, 9: 82-9. |
| [85] |
Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther, 2007, 15: 651-9. |
| [86] |
Martuza RL. Conditionally replicating herpes vectors for cancer therapy. J Clin Invest, 2000, 105: 841-6. |
| [87] |
Ripa I, Andreu S, López-Guerrero JA, et al. Interplay between autophagy and herpes simplex virus type 1: ICP34.5, one of the main actors. Int J Mol Sci, 2022, 23: 13643. |
| [88] |
Mao H, Rosenthal KS. Strain-dependent structural variants of herpes simplex virus type 1 ICP34.5 determine viral plaque size, efficiency of glycoprotein processing, and viral release and neuroinvasive disease potential. J Virol, 2003, 77: 3409-17. |
| [89] |
Cassady KA, Gross M, Roizman B. The herpes simplex virus US11 protein effectively compensates for the γ1(34.5) gene if present before activation of protein kinase R by precluding its phosphorylation and that of the α subunit of eukaryotic translation initiation factor 2. J Virol, 1998, 72: 8620-6. |
| [90] |
Goldsmith K, Chen W, Johnson DC, et al. Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response. J Exp Med, 1998, 187: 341-8. |
| [91] |
Fukuhara H, Takeshima Y, Todo T. Triple-mutated oncolytic herpes virus for treating both fast- and slow-growing tumors. Cancer Sci, 2021, 112: 3293-301. |
| [92] |
Longo SL, Griffith C, Glass A, et al. Development of an oncolytic herpes simplex virus using a tumor-specific HIF-responsive promoter. Cancer Gene Ther, 2011, 18: 123-34. |
| [93] |
Miyatake SI, Tani S, Feigenbaum F, et al. Hepatoma-specific antitumor activity of an albumin enhancer/promoter regulated herpes simplex virus in vivo. Gene Ther, 1999, 6: 564-72. |
| [94] |
Yamamura H, Hashio M, Noguchi M, et al. Identification of the transcriptional regulatory sequences of human calponin promoter and their use in targeting a conditionally replicating herpes vector to malignant human soft tissue and bone tumors. Cancer Res, 2001, 61: 3969-77. |
| [95] |
Kuroda T, Rabkin SD, Martuza RL. Effective treatment of tumors with strong β-catenin/T-cell factor activity by transcriptionally targeted oncolytic herpes simplex virus vector. Cancer Res, 2006, 66: 10127-35. |
| [96] |
Mullen JT, Kasuya H, Yoon SS, et al. Regulation of herpes simplex virus 1 replication using tumor-associated promoters. Ann Surg, 2002, 236: 502-13. |
| [97] |
Nakashima H, Nguyen T, Kasai K, et al. Toxicity and efficacy of a novel GADD34-expressing oncolytic HSV-1 for the treatment of experimental glioblastoma. Clin Cancer Res, 2018, 24: 2574-84. |
| [98] |
Cheng L, Jiang H, Fan J, et al. A novel oncolytic herpes simplex virus armed with the carboxyl-terminus of murine MyD116 has enhanced anti-tumour efficacy against human breast cancer cells. Oncol Lett, 2018, 15: 7046-52. |
| [99] |
Shen Y, Bai X, Zhang Q, et al. Oncolytic virus VG161 in refractory hepatocellular carcinoma. Nature, 2025, 641: 503-11. |
| [100] |
Shen Y, Song W, Lin D, et al. VG161 activates systemic antitumor immunity in pancreatic cancer models as a novel oncolytic herpesvirus expressing multiple immunomodulatory transgenes. J Med Virol, 2023, 95: e28108. |
| [101] |
Parker JN, Meleth S, Hughes KB, et al. Enhanced inhibition of syngeneic murine tumors by combinatorial therapy with genetically engineered HSV-1 expressing CCL2 and IL-12. Cancer Gene Ther, 2005, 12: 359-68. |
| [102] |
Chaurasiya S, Fong Y, Warner SG. Oncolytic virotherapy for cancer: clinical experience. Biomedicines, 2021, 9: 419. |
| [103] |
Tian L, Xu B, Chen Y, et al. Specific targeting of glioblastoma with an oncolytic virus expressing a cetuximab-CCL5 fusion protein via innate and adaptive immunity. Nat Cancer, 2022, 3: 1318-35. |
| [104] |
Tian C, Liu J, Zhou H, et al. Enhanced anti-tumor response elicited by a novel oncolytic HSV-1 engineered with an anti-PD-1 antibody. Cancer Lett, 2021, 518: 49-58. |
| [105] |
Haines BB, Denslow A, Grzesik P, et al. ONCR-177, an oncolytic HSV-1 designed to potently activate systemic antitumor immunity. Cancer Immunol Res, 2021, 9: 291-308. |
| [106] |
Huang S, Hu H, Tang G, et al. An oncolytic herpes simplex virus type 1 strain expressing a single-chain variable region antibody fragment against PD-1 and a PI3K inhibitor synergize to elicit antitumor immunity in ovarian cancer. Arch Virol, 2023, 168: 128. |
| [107] |
Ju F, Luo Y, Lin C, et al. Oncolytic virus expressing PD-1 inhibitors activates a collaborative intratumoral immune response to control tumor and synergizes with CTLA-4 or TIM-3 blockade. J Immunother Cancer, 2022, 10: e004762. |
| [108] |
Shimizu K, Kahramanian A, Jabbar MADA, et al. Photodynamic augmentation of oncolytic virus therapy for central nervous system malignancies. Cancer Lett, 2023, 572: 216363. |
| [109] |
Davison AJ, Benkő M, Harrach B. Genetic content and evolution of adenoviruses. J Gen Virol, 2003, 84: 2895-908. |
| [110] |
Enow JA, Sheikh HI, Rahman MM. Tumor tropism of DNA viruses for oncolytic virotherapy. Viruses, 2023, 15: 2262. |
| [111] |
Kaplan JM. Adenovirus-based cancer gene therapy. Curr Gene Ther, 2005, 5: 595-605. |
| [112] |
Ehrke-Schulz E, Zhang W, Schiwon M, et al. Cloning and large-scale production of high-capacity adenoviral vectors based on the human adenovirus type 5. J Vis Exp, 2016(107): e52894. |
| [113] |
Hidalgo P, Gonzalez RA. Formation of adenovirus DNA replication compartments. FEBS Lett, 2019, 593: 3518-30. |
| [114] |
Lynch JP 3rd, Fishbein M, Echavarria M. Adenovirus. Semin Respir Crit Care Med, 2011, 32: 494-511. |
| [115] |
Zhang QN, Li Y, Zhao Q, et al. Recombinant human adenovirus type 5 (Oncorine) reverses resistance to immune checkpoint inhibitor in a patient with recurrent non-small cell lung cancer: a case report. Thorac Cancer, 2021, 12: 1617-9. |
| [116] |
Wei D, Xu J, Liu XY, et al. Fighting cancer with viruses: oncolytic virus therapy in China. Hum Gene Ther, 2018, 29: 151-9. |
| [117] |
Monsen RC, DeLeeuw L, Dean WL, et al. The hTERT core promoter forms three parallel G-quadruplexes. Nucleic Acids Res, 2020, 48: 5720-34. |
| [118] |
Kawashima T, Kagawa S, Kobayashi N, et al. Telomerase-specific replication-selective virotherapy for human cancer. Clin Cancer Res, 2004, 10: 285-92. |
| [119] |
Yano S, Tazawa H, Hashimoto Y, et al. Mechanistic analysis of a novel, telomerase-specific oncolytic adenovirus targeting human gastric cancer stem cells. J Clin Oncol, 2010, 28: e13626. |
| [120] |
Araki H, Tazawa H, Kanaya N, et al. Oncolytic virus-mediated p53 overexpression promotes immunogenic cell death and efficacy of PD-1 blockade in pancreatic cancer. Mol Ther Oncolytics, 2022, 27: 3-13. |
| [121] |
Zhang KJ, Wang YG, Cao X, et al. Potent antitumor effect of interleukin-24 gene in the survivin promoter and retinoblastoma double-regulated oncolytic adenovirus. Hum Gene Ther, 2009, 20: 818-30. |
| [122] |
Li HJ, Everts M, Yamamoto M, et al. Combined transductional untargeting/retargeting and transcriptional restriction enhances adenovirus gene targeting and therapy for hepatic colorectal cancer tumors. Cancer Res, 2009, 69: 554-64. |
| [123] |
Takahashi M, Sato T, Sagawa T, et al. E1B-55K-deleted adenovirus expressing E1A-13S by AFP-enhancer/promoter is capable of highly specific replication in AFP-producing hepatocellular carcinoma and eradication of established tumor. Mol Ther, 2002, 5: 627-34. |
| [124] |
Xu C, Sun Y, Wang Y, et al. CEA promoter-regulated oncolytic adenovirus-mediated Hsp70 expression in immune gene therapy for pancreatic cancer. Cancer Lett, 2012, 319: 154-63. |
| [125] |
Huang H, Liu Y, Liao W, et al. Oncolytic adenovirus programmed by synthetic gene circuit for cancer immunotherapy. Nat Commun, 2019, 10: 4801. |
| [126] |
Parato KA, Breitbach CJ, Le Boeuf F, et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol Ther, 2012, 20: 749-58. |
| [127] |
Ge Y, Wang H, Ren J, et al. Oncolytic vaccinia virus delivering tethered IL-12 enhances antitumor effects with improved safety. J Immunother Cancer, 2020, 8: e000710. |
| [128] |
Shakiba Y, Vorobyev PO, Yusubalieva GM, et al. Oncolytic therapy with recombinant vaccinia viruses targeting the interleukin-15 pathway elicits a synergistic response. Mol Ther Oncolytics, 2023, 29: 158-68. |
| [129] |
Yang M, Giehl E, Feng C, et al. IL-36γ-armed oncolytic virus exerts superior efficacy through induction of potent adaptive antitumor immunity. Cancer Immunol Immunother, 2021, 70: 2467-81. |
| [130] |
Chen T, Ding X, Liao Q, et al. IL-21 arming potentiates the anti-tumor activity of an oncolytic vaccinia virus in monotherapy and combination therapy. J Immunother Cancer, 2021, 9: e001647. |
| [131] |
Li J, O'Malley M, Urban J, et al. Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Mol Ther, 2011, 19: 650-7. |
| [132] |
Liu Z, Ravindranathan R, Li J, et al. CXCL11-armed oncolytic poxvirus elicits potent antitumor immunity and shows enhanced therapeutic efficacy. Oncoimmunology, 2015, 5: e1091554. |
| [133] |
Chon HJ, Lee WS, Yang H, et al. Tumor microenvironment remodeling by intratumoral oncolytic vaccinia virus enhances the efficacy of immune-checkpoint blockade. Clin Cancer Res, 2019, 25: 1612-23. |
| [134] |
Wang G, Kang X, Chen KS, et al. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses. Nat Commun, 2020, 11: 1395. |
| [135] |
Zuo S, Wei M, Xu T, et al. An engineered oncolytic vaccinia virus encoding a single-chain variable fragment against TIGIT induces effective antitumor immunity and synergizes with PD-1 or LAG-3 blockade. J Immunother Cancer, 2021, 9: e002843. |
| [136] |
Park AK, Fong Y, Kim SI, et al. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci Transl Med, 2020, 12: eaaz1863. |
| [137] |
Aalipour A, Le Boeuf F, Tang M, et al. Viral delivery of CAR targets to solid tumors enables effective cell therapy. Mol Ther Oncolytics, 2020, 17: 232-40. |
| [138] |
Peng KW, Donovan KA, Schneider U, et al. Oncolytic measles viruses displaying a single-chain antibody against CD38, a myeloma cell marker. Blood, 2003, 101: 2557-62. |
| [139] |
Bucheit AD, Kumar S, Grote DM, et al. An oncolytic measles virus engineered to enter cells through the CD20 antigen. Mol Ther, 2003, 7: 62-72. |
| [140] |
Ong HT, Trejo TR, Pham LD, et al. Intravascularly administered RGD-displaying measles viruses bind to and infect neovessel endothelial cells in vivo. Mol Ther, 2009, 17: 1012-21. |
| [141] |
Friedrich K, Hanauer JR, Prüfer S, et al. DARPin-targeting of measles virus: unique bispecificity, effective oncolysis, and enhanced safety. Mol Ther, 2013, 21: 849-59. |
| [142] |
Mühlebach MD, Schaser T, Zimmermann M, et al. Liver cancer protease activity profiles support therapeutic options with matrix metalloproteinase-activatable oncolytic measles virus. Cancer Res, 2010, 70: 7620-9. |
| [143] |
Tahara M, Takishima Y, Miyamoto S, et al. Photocontrollable mononegaviruses. Proc Natl Acad Sci U S A, 2019, 116: 11587-9. |
| [144] |
Ketzer P, Kaufmann JK, Engelhardt S, et al. Artificial riboswitches for gene expression and replication control of DNA and RNA viruses. Proc Natl Acad Sci U S A, 2014, 111: E554-62. |
| [145] |
Leber MF, Bossow S, Leonard VH, et al. MicroRNA-sensitive oncolytic measles viruses for cancer-specific vector tropism. Mol Ther, 2011, 19: 1097-106. |
| [146] |
Grossardt C, Engeland CE, Bossow S, et al. Granulocyte-macrophage colony-stimulating factor-armed oncolytic measles virus is an effective therapeutic cancer vaccine. Hum Gene Ther, 2013, 24: 644-54. |
| [147] |
Veinalde R, Grossardt C, Hartmann L, et al. Oncolytic measles virus encoding interleukin-12 mediates potent antitumor effects through T cell activation. Oncoimmunology, 2017, 6: e1285992. |
| [148] |
Engeland CE, Grossardt C, Veinalde R, et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol Ther, 2014, 22: 1949-59. |
| [149] |
Liu H, Xue YC, Deng H, et al. MicroRNA modification of coxsackievirus B3 decreases its toxicity, while retaining oncolytic potency against lung cancer. Mol Ther Oncolytics, 2020, 16: 207-18. |
| [150] |
Zhong L, Gan L, Wang B, et al. Hyperacute rejection-engineered oncolytic virus for interventional clinical trial in refractory cancer patients. Cell, 2025, 188: 1119-36. |
| [151] |
Shalhout SZ, Miller DM, Emerick KS, et al. Therapy with oncolytic viruses: progress and challenges. Nat Rev Clin Oncol, 2023, 20: 160-77. |
| [152] |
Hill C, Carlisle R. Achieving systemic delivery of oncolytic viruses. Expert Opin Drug Deliv, 2019, 16: 607-20. |
| [153] |
Chen Y, Chen X, Bao W, et al. An oncolytic virus-T cell chimera for cancer immunotherapy. Nat Biotechnol, 2024, 42: 1876-87. |
| [154] |
Fares J, Ahmed AU, Ulasov IV, et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: a first-in-human, phase 1, dose-escalation trial. Lancet Oncol, 2021, 22: 1103-14. |
| [155] |
Ahmed AU, Thaci B, Tobias AL, et al. A preclinical evaluation of neural stem cell-based cell carrier for targeted antiglioma oncolytic virotherapy. J Natl Cancer Inst, 2013, 105: 968-77. |
| [156] |
Castleton A, Dey A, Beaton B, et al. Human mesenchymal stromal cells deliver systemic oncolytic measles virus to treat acute lymphoblastic leukemia in the presence of humoral immunity. Blood, 2014, 123: 1327-35. |
| [157] |
Leoni V, Gatta V, Palladini A, et al. Systemic delivery of HER2-retargeted oncolytic-HSV by mesenchymal stromal cells protects from lung and brain metastases. Oncotarget, 2015, 6: 34774-87. |
| [158] |
Rincón E, Cejalvo T, Kanojia D, et al. Mesenchymal stem cell carriers enhance antitumor efficacy of oncolytic adenoviruses in an immunocompetent mouse model. Oncotarget, 2017, 8: 45415-31. |
| [159] |
Draganov DD, Santidrian AF, Minev I, et al. Delivery of oncolytic vaccinia virus by matched allogeneic stem cells overcomes critical innate and adaptive immune barriers. J Transl Med, 2019, 17: 100. |
| [160] |
Jazowiecka-Rakus J, Sochanik A, Rusin A, et al. Myxoma virus-loaded mesenchymal stem cells in experimental oncolytic therapy of murine pulmonary melanoma. Mol Ther Oncolytics, 2020, 18: 335-50. |
| [161] |
Wu Q, Huang H, Sun M, et al. Inhibition of tumor metastasis by liquid-nitrogen-shocked tumor cells with oncolytic viruses infection. Adv Mater, 2023, 35: e2212210. |
| [162] |
Wen P, Huang H, Zhang R, et al. Coacervate vesicles assembled by liquid-liquid phase separation improve delivery of biopharmaceuticals. Nat Chem, 2025, 17: 279-88. |
 2025, Vol. 37
2025, Vol. 37 

 刘涛,北京大学博雅特聘教授,国家杰青、国家优青、北京市杰青基金获得者。任北京大学药学院分子与细胞药理系主任。担任Journal of Molecular Biology和Chinese Chemical Letter杂志编委。任中国医药生物技术协会合成生物学分会委员、中国生物工程学会合成生物学分会青年工作组委员。获中国药学会生物医药青年奖、拜耳学者奖、屠呦呦青年学者奖、北京大学王选青年学者奖等。以通讯作者身份在Nat Chem、Nat Chem Biol、Mol Cell、Nat Commun、Sci Adv、Chem、JACS、ACIE等发表论文30余篇。研究集中在基于细胞工程的蛋白质精准化学修饰技术,通过非天然氨基酸介导的蛋白质化学修饰实现蛋白质、病毒以及细胞等生物技术药物的升级换代与创新;
刘涛,北京大学博雅特聘教授,国家杰青、国家优青、北京市杰青基金获得者。任北京大学药学院分子与细胞药理系主任。担任Journal of Molecular Biology和Chinese Chemical Letter杂志编委。任中国医药生物技术协会合成生物学分会委员、中国生物工程学会合成生物学分会青年工作组委员。获中国药学会生物医药青年奖、拜耳学者奖、屠呦呦青年学者奖、北京大学王选青年学者奖等。以通讯作者身份在Nat Chem、Nat Chem Biol、Mol Cell、Nat Commun、Sci Adv、Chem、JACS、ACIE等发表论文30余篇。研究集中在基于细胞工程的蛋白质精准化学修饰技术,通过非天然氨基酸介导的蛋白质化学修饰实现蛋白质、病毒以及细胞等生物技术药物的升级换代与创新; 王永,北京大学副研究员。以第一(含共同)/通讯(含共同)作者身份在Nat Commun、Angew Chem Int Ed、Nano Lett、Bioorg Med Chem、Methods Mol Biol等发表论文多篇。主要研究方向为蛋白质的精准化学修饰,致力于构建新型生物正交反应工具,推动蛋白质药物的功能优化与创新应用,研究内容聚焦于抗体-药物偶联(ADC)和细菌治疗等领域
王永,北京大学副研究员。以第一(含共同)/通讯(含共同)作者身份在Nat Commun、Angew Chem Int Ed、Nano Lett、Bioorg Med Chem、Methods Mol Biol等发表论文多篇。主要研究方向为蛋白质的精准化学修饰,致力于构建新型生物正交反应工具,推动蛋白质药物的功能优化与创新应用,研究内容聚焦于抗体-药物偶联(ADC)和细菌治疗等领域