唾液蛋白作为各种植食性昆虫的唾液组分,可分解植物有毒物质,激发或抑制植物的抗性反应并调节昆虫的取食[1]。由于不同昆虫的唾液蛋白种类和丰度不同,昆虫在取食不同的寄主植物时进化出了不同的取食策略[2]。昆虫的取食也是虫媒病毒能否成功传播的关键。植物病毒可通过改变介体昆虫的唾液蛋白分泌操控植物防御反应,便利昆虫取食,实现病毒的有效传播[3-4]。近年来,关于介体昆虫唾液蛋白调控植物防御反应和病毒传播的机制已成为研究热点,但尚缺乏对该类研究的专题论述。本文以刺吸式口器介体昆虫唾液蛋白调控植物防御反应的分子机制为出发点,探讨唾液蛋白与昆虫取食及病毒传播的关系,从昆虫取食行为和植物防御反应两个方面阐述唾液蛋白的功能。
1 昆虫唾液种类及唾液蛋白的功能刺吸式口器昆虫的唾液分为胶状唾液和水状唾液。胶状唾液又称鞘状唾液,分泌后会凝结成唾液鞘,主要成分为蛋白质、磷脂和碳水化合物,起固定和保护口针的作用[2]。鞘状唾液中的唾液鞘蛋白(salivary sheath protein)和类黏蛋白(mucin-like protein)可促进唾液鞘的形成,并与寄主互作,提高昆虫适应性[5-6]。水状唾液的功能和成分相对复杂,包含多种酶类及结合蛋白。其中,多酚氧化酶、过氧化氢酶和氧化还原酶等解毒酶类,有助于昆虫分解植物有毒次生物质和抑制植物防御反应[7]。唾液中降解植物组织的水解酶类,如果胶酶、淀粉酶、纤维素酶等,可促进昆虫口针穿刺和吸收营养物质[8-9]。唾液中的蛋白水解酶类可识别不同的氨基酸作用位点,以增加额外的氨基酸摄入[8]。
从唾液蛋白的功能角度而言,一些唾液蛋白可进入寄主细胞中操纵植物的防御反应,被称为唾液效应子,因此唾液腺又被称为昆虫效应子的合成“工厂”[10]。一些唾液蛋白也可作为激发子,被寄主植物识别进而诱发自身的防御反应,对抗昆虫的取食[11-13]。随着高通量测序技术和蛋白质组学技术的发展,科研人员已鉴定分析了蚜虫、飞虱、蝽类等多种刺吸式口器昆虫的唾液成分,为探索唾液蛋白在昆虫-植物互作中的功能奠定了基础[14-16]。
2 唾液蛋白调控植物防御反应刺吸式口器的昆虫在刺探和取食过程中会对寄主植物造成损伤。而在昆虫与植物长期的协同进化过程中,植物进化出了防御系统来对抗昆虫的攻击,植食性昆虫相关分子模式(herbivore-associated molecular pattern, HAMP) 会识别并激活植物HAMP,诱导免疫反应(HAMP-triggered immunity, HTI)。这类防御反应是植物抵挡植食性昆虫取食的第一道防线。为了顺利取食,昆虫会通过分泌唾液效应子削弱植物的防御反应。植物自身的特异性抗性基因(R)会识别昆虫的效应子,从而激活效应子诱导的免疫反应(effector-triggered immunity, ETI)来特异地抵御昆虫取食。下面针对唾液激发子和唾液效应子,详细阐述唾液蛋白调控植物防御反应的分子机制,其大致过程如图 1所示。
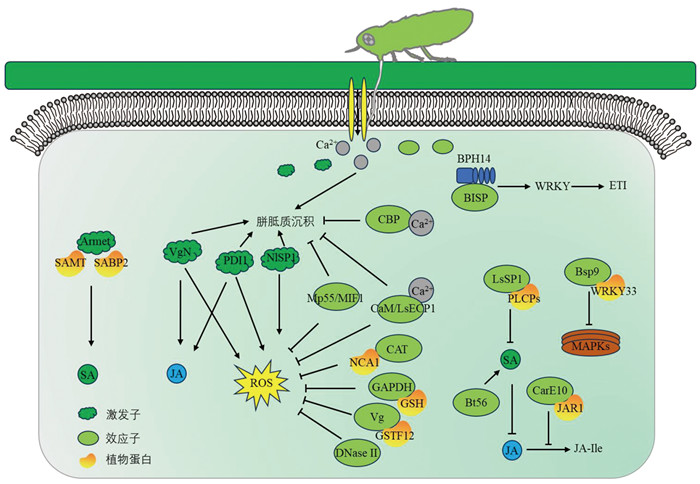
|
刺吸式昆虫的唾液激发子会诱发ROS爆发、植物激素的合成等。蚜虫唾液激发子Armet调控SAMT和SABP2的表达,从而促进SA的合成。褐飞虱唾液激发子VgN、PDI1和NlSP1诱导ROS爆发、JA合成以及胼胝质的积累。昆虫也会分泌唾液效应子调控植物防御反应。蚜虫唾液效应子Mp55和MIF1抑制寄主植物ROS爆发和胼胝质积累。叶蝉唾液效应子CAT、GAPDH和Vg分别与水稻蛋白NCA1、GSH和GSTF2互作,降低H2O2水平,抑制ROS爆发。灰飞虱唾液DNase Ⅱ分解细胞外DNA,抑制ROS爆发。叶蝉唾液效应子CBP和灰飞虱唾液效应子CaM、LsECP1调控水稻Ca2+信号,抑制H2O2的产生。叶蝉唾液效应子CarE与水稻JAR1互作抑制JA-Ile的合成。灰飞虱唾液效应子LsSP1吸附水稻PLCPs,抑制SA信号抗性反应。蚜虫唾液效应子Bt56促进SA的合成,抑制JA信号通路的抗性反应。烟粉虱唾液效应子Bsp9与WRKY33互作,抑制MAPK信号转导途径的抗性反应。褐飞虱唾液效应子被水稻NLRs蛋白BPH14识别,触发ETI。 图 1 唾液蛋白调控植物防御反应的示意图 |
刺吸式口器昆虫刺探植物表皮细胞时,会分泌果胶酶、淀粉酶、纤维素酶等细胞壁降解酶类,疏松植物细胞壁,以便利口针的插入,细胞壁降解产物可作为损伤相关分子模式(damage associated molecular pattern, DAMP)信号,被植物模式识别受体(pattern- recognition receptor, PRR)识别触发丝裂原活化蛋白激酶(mitogen activated protein kinase, MAPK)信号级联反应、活性氧(reactive oxygen species, ROS)等防御反应[2]。昆虫取食韧皮部还会改变植物细胞质和液泡膜间的Ca2+流动。伤口部位的Ca2+外泄会引起筛管阻塞,引发胼胝质沉积等多种防御反应,从而抵抗昆虫取食[17]。
植物激素调控的免疫反应是植物的重要防御手段,昆虫唾液激发子还可以促进植物激素的合成[18]。如豌豆蚜(Acyrthosiphon pisum)的唾液蛋白Armet可以通过调控水杨酸(salicylic acid, SA)代谢关键基因水杨酸甲基转移酶(salicylic acid methyltransferase, SAMT)和水杨酸结合蛋白2 (salicylic acid binding protein 2, SABP2)的表达,实现SA的积累,诱导植物抗性[19-20]。昆虫分泌的唾液激发子被PRR识别触发植物HTI反应,激活下游的MAPK信号级联反应,诱导产生SA、茉莉酸(jasmonic acid, JA)等植物激素,造成ROS爆发及其他防御物质的积累[21]。褐飞虱唾液蛋白1 (Nilaparvata lugens Stål salivary protein 1, NlSP1)和褐飞虱的唾液蛋白质二硫键异构酶1 (protein disulfide isomerase 1, PDI1)、卵黄原蛋白(vitellogenin, Vg) N端亚基可作为激发子诱导水稻ROS爆发、胼胝质积累和细胞死亡等防御和免疫反应[11-13]。
2.2 唾液效应子抑制植物防御反应 2.2.1 唾液效应子抑制Ca2+信号和ROS爆发人们通过应用生物信息学及蛋白质组学技术,已鉴定出不同昆虫的唾液效应子,如桃蚜(Myzus persicae)的巨噬细胞移动抑制因子(migration inhibitory factor 1, MIF1)和Mp55可作为唾液效应子,促进蚜虫的取食[22-23]。褐飞虱唾液中具有Ca2+结合活性的EF-hand蛋白SEF1 (salivary EF-hand 1)可作为效应子抑制Ca2+和H2O2含量升高[24]。灰飞虱(Laodelphax striatellus)唾液钙调蛋白(calmodulin, CaM)和Ca2+结合蛋白(LsECP1)调控水稻Ca2+信号,抑制H2O2的产生[25-26]。唾液DNA酶Ⅱ (DNase Ⅱ)通过降解细胞外DNA,抑制细胞外DNA激发植物的ROS,促进昆虫取食[27]。叶蝉也可分泌多种唾液效应子抑制水稻抗虫反应,促进自身取食。例如,唾液过氧化氢酶(catalase, CAT)通过与寄主水稻分子伴侣NCA1 (NO CATALASE ACTIVITY 1)互作,增强了分解H2O2的能力,从而抑制ROS,促进昆虫取食[28]。叶蝉Vg也可以作为唾液蛋白被分泌到水稻韧皮部,抑制水稻H2O2爆发[29]。叶蝉唾液中的甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase, GAPDH)进入水稻韧皮部后通过还原H2O2,抑制植物抗虫反应,而水稻谷胱甘肽(glutathione, GSH)通过抑制GAPDH过度氧化,以减轻GAPDH氧化产物对细胞的毒性,促进昆虫可持续性地取食[30]。
2.2.2 唾液效应子抑制植物激素信号途径昆虫唾液效应子还可抑制植物激素JA和SA信号途径。褐飞虱气味结合蛋白OBP11 (odorant-binding protein 11)可随唾液分泌至水稻,抑制水稻JA信号途径的防御反应[31]。灰飞虱的唾液鞘蛋白LsSP1可特异性地结合在类黏蛋白周围,以避免其被植物防御系统识别;LsSP1还可吸附水稻半胱氨酸蛋白酶,破坏其与SA通路的正反馈调控回路,进而抑制水稻免疫反应[32]。叶蝉唾液羧酸酯酶CarE10 (carboxylesterase 10)通过抑制茉莉酸异亮氨酸(jasmonic acid-isoleucine, JA-Ile)的合成抑制水稻防御反应[33]。JA和SA信号途径之间的相互拮抗也为昆虫在取食时逃逸寄主植物的抗性反应提供可乘之机[34]。B型烟粉虱(Bemisia tabaci type B)的取食可诱导SA信号通路相关基因的表达,并抑制JA信号通路相关基因的表达,推测其唾液蛋白可能利用JA和SA之间的拮抗,抑制JA抗性反应[35]。此外,一些粉虱和蚜虫的唾液蛋白(Bt56、Armet)也能通过干扰植物激素之间的相互作用来抑制植物防御反应[20, 36]。
2.2.3 唾液效应子抑制韧皮部阻塞和胼胝质积累昆虫口针经过一系列刺探后成功穿刺至韧皮部,从而建立取食通道。刺吸式口器昆虫长时间取食韧皮部会干扰韧皮部的光合作用,从而影响能量源的运输,于是植物激活韧皮部筛管分子阻塞机制,阻止汁液的流失,阻碍昆虫的取食。
韧皮部阻塞反应分为两个过程,植物感知损伤后,首先合成P蛋白快速密封筛管伤口,随后形成胼胝质堵塞筛管。P蛋白由筛管分子阻塞(sieve element occlusion, SEO)基因编码,其纤维细丝在筛孔聚集阻塞筛孔,防止损伤部位内容物的流失[37]。一些P蛋白具有收缩和发散两种构象状态,在pH和Ca2+浓度的影响下,P蛋白在两种构象间可逆转变,从而定向阻塞筛孔[38]。为保证昆虫正常取食,唾液蛋白可干扰韧皮部阻塞反应,促进昆虫取食。蚜虫的一些唾液蛋白可与Ca2+结合,阻止取食损伤造成Ca2+流向韧皮部,抑制P蛋白的聚集,便利了蚜虫取食[2]。
胼胝质是由胼胝质合成酶(callose synthase, CALS)合成的β-1, 3-葡聚糖构成[39]。昆虫口针刺入筛管时,质外体Ca2+流入筛管中与CALS结合,引起胼胝质沉积在筛板处[40]。因此,胼胝质也是植物抵御刺吸式口器昆虫取食的重要“武器”。而昆虫的胶状唾液可填充口针穿刺造成的伤口防止Ca2+外泄[41],水状唾液中的Ca2+结合蛋白可调节Ca2+浓度延缓胼胝质的积累[2, 17],保证取食行为的顺利进行。叶蝉唾液钙离子结合蛋白(calcium binding protein, CBP)可结合Ca2+,降低游离的Ca2+浓度,延缓胼胝质的积累;而抑制电光叶蝉唾液腺中CBP的表达使电光叶蝉取食时诱导水稻产生更高含量的Ca2+,触发韧皮部胼胝质的沉积,导致电光叶蝉取食困难而增加刺探取食频率[42]。
2.2.4 唾液效应子调控NLRs介导的抗性反应植物的核苷酸结合和富亮氨酸重复域受体(nucleotide binding and leucine-rich repeat domain receptor, NLR)蛋白也能够特异性识别昆虫唾液效应子,诱发ETI反应[43]。目前,人们已经发现多种抵抗韧皮部取食昆虫的植物R基因,但是对其作用机制尚不清楚[18]。有研究报道番茄R基因Mi-1不但可以抵御南方根结线虫(Meloidogyne incognita),同时可诱导产生对蚜虫和粉虱的抗性[44]。尽管已经明确发现NLRs对韧皮部取食的昆虫具有抗性,但是只有与水稻NLR受体BPH14互作的唾液蛋白(BPH14-interacting salivary protein, BISP)是目前已知的被NLR识别的唾液效应子。水稻NLR受体BPH14特异性地识别BISP后,通过与WRKY转录因子互作,增强WRKY活性,促进抗性相关基因的表达,从而有效抵御褐飞虱的取食[45-46]。值得注意的是,BISP不仅触发了BPH14介导的抗性反应,还可以激活选择性自噬,并且通过自噬降解BISP,以限制防御反应过度激活[46]。
3 唾液蛋白对植物病毒传播的影响取食是昆虫获毒和传毒的主要途径,那么调控昆虫取食行为的唾液蛋白则在传毒过程中扮演重要角色。非持久性病毒经昆虫取食后与位于口针的受体蛋白结合并停留在口针处[47],待昆虫取食时随唾液一起分泌到植物中。目前尚不清楚唾液蛋白、受体蛋白和病毒粒体之间的相互联系[48]。持久性病毒经昆虫长时间取食韧皮部进入昆虫体内,并经过循回到达唾液腺,在昆虫取食时伴随唾液一起释放至植物,完成传播[49]。虽然非持久性和持久性病毒都依赖昆虫唾液传播,但唾液蛋白对于两种类型病毒传播的影响完全不同,持久性病毒在唾液腺中可参与调控唾液蛋白的合成和分泌以便自身传播,而非持久性病毒不在昆虫体内循回,在传播过程被动受唾液的影响[50]。下面分别介绍昆虫唾液蛋白对非持久性病毒和持久性病毒传播的影响。
3.1 唾液蛋白对非持久性病毒传播的影响非持久性病毒会在一定程度上对介体昆虫的生理生化造成影响,虽然其对介体昆虫唾液蛋白分泌的调控机制尚不明确,但是唾液蛋白可通过触发植物的防御反应间接地抑制非持久性病毒的传播[50]。非持久性病毒的传播则是通过调控植物的生长状态,操纵介体昆虫的寄主趋向和取食行为来实现的[49, 51-52]。
昆虫取食寄主植物时,会向唾液分泌细胞壁降解酶类蛋白,疏松细胞壁的机械阻力,方便取食。较低的细胞壁机械压力也促进病毒进入植物细胞完成侵染[53]。昆虫唾液蛋白诱导产生的细胞壁降解产物可作为DAMP信号,诱导植物产生防御反应,如ROS等[2]。唾液蛋白诱导的植物防御反应可作为重要的抗性因子抵抗病毒的侵染[54],也可以作为促进因子帮助病毒复制[55]。一些唾液蛋白也可以通过调控质外体的酸碱平衡,疏松质外体结构,促进病毒侵染。蚜虫唾液碳酸酐酶Ⅱ (carbonic anhydrase Ⅱ, CA-Ⅱ)通过增强植物质外体酸化,加快植物囊泡运输果胶,以强化细胞壁,促进黄瓜花叶病毒(cucumber mosaic virus, CMV)和芜菁花叶病毒(turnip mosaic virus, TuMV)通过囊泡实现细胞间转运,进而帮助病毒传播[50]。
马铃薯X病毒(potato virus X, PVX)和CMV等植物病毒可以被植物免疫受体识别并触发病原微生物相关的分子模式激活的免疫反应(pathogen-associated molecular pattern-triggered immunity, PTI)[56-57]。为保证取食行为的顺利进行,昆虫唾液蛋白可作为效应子抑制PTI反应,但唾液效应子又可被寄主植物的R基因所识别从而诱发更为强烈的ETI反应[23]。而一些有效抗虫的R基因,如Mi-1.2、Vat 等,同样可以识别病毒因子,进而产生进一步的抗病毒反应[58-59]。一些昆虫唾液蛋白激发的HTI也可抑制植物病毒的侵染。马铃薯长管蚜(Macrosiphum euphorbiae)共生菌分泌的分子伴侣GroEL蛋白可以作为唾液激发子激发植物受体油菜素内酯不敏感受体激酶(brassinosteroid insensitive-associated kinase 1, BAK1)介导的PTI反应[60],而BAK1依赖的抗性反应还可以抑制烟草花叶病毒(tobacco mosaic virus, TMV)和芜菁皱缩病毒(turnip crinkle virus, TCV)的积累[61]。
3.2 唾液蛋白对持久性病毒传播的影响持久性病毒的传播介体包括叶蝉、飞虱、蚜虫和蓟马等。持久性病毒在介体昆虫体内循回过程中,从肠腔进入血淋巴或其他组织,最后进入唾液腺,这一过程面临着多种传播屏障——中肠侵入屏障、中肠释放屏障、唾液腺侵染屏障、唾液腺释放屏障及垂直传播屏障[62]。唾液腺释放屏障是虫媒病毒向植物传播的最后一道屏障。唾液腺中病毒的侵染和释放必然会影响唾液蛋白的产生和分泌,进而影响昆虫的取食行为。水稻瘤矮病毒(rice gall dwarf virus, RGDV)作为典型的持久性传播病毒,调控多种唾液蛋白的表达和分泌。RGDV的非结构蛋白Pns11形成的囊泡可以包裹唾液蛋白CarE10并将其运输到唾液腔中,促进CarE10分泌到水稻韧皮部[33]。RGDV侵染唾液腺激发H2O2的合成,高水平的H2O2诱导CAT的表达,使唾液CAT含量升高[28]。叶蝉GAPDH通过搭载外泌体进入唾液腔中,RGDV的侵染促进外泌体的产生,进而促进GAPDH随外泌体分泌到水稻韧皮部[30]。水稻矮缩病毒(rice dwarf virus, RDV)通过搭载外泌体穿过唾液顶端质膜进入唾液腔,RDV的外壳蛋白P2通过互作将Vg劫持到外泌体中,促进Vg搭载外泌体分泌水稻韧皮部[29]。RGDV或RDV通过调控唾液蛋白CarE10、CAT、Vg和GAPDH的分泌,抑制植物JA信号途径或H2O2诱发的防御反应,促进昆虫的取食,进而促进病毒的传播[28-30, 33]。电光叶蝉唾液蛋白CBP与RGDV的Pns11蛋白竞争性结合肌动蛋白Actin,抑制Pns11形成的丝状结构通过Actin穿过顶端质膜进入唾液腔,为保证Pns11的丝状结构顺利进入唾液腔,RGDV抑制CBP的表达和分泌,使介体在取食时诱导水稻产生更高水平的Ca2+,从而触发韧皮部胼胝质的沉积,影响昆虫的取食,促进病毒的传播[42]。番茄黄化曲叶病毒(tomato yellow leaf curl virus, TYLCV)诱导烟粉虱表达唾液蛋白Bsp9,从而与抗性反应相关转录因子WRKY33互作,抑制MAPK信号转导途径的抗性反应,有利于昆虫的取食和病毒的传播[10, 63-64]。因此,持久性传播病毒可以通过调控唾液蛋白的分泌调控昆虫的取食行为,影响病毒自身的传播[65-66](图 2)。
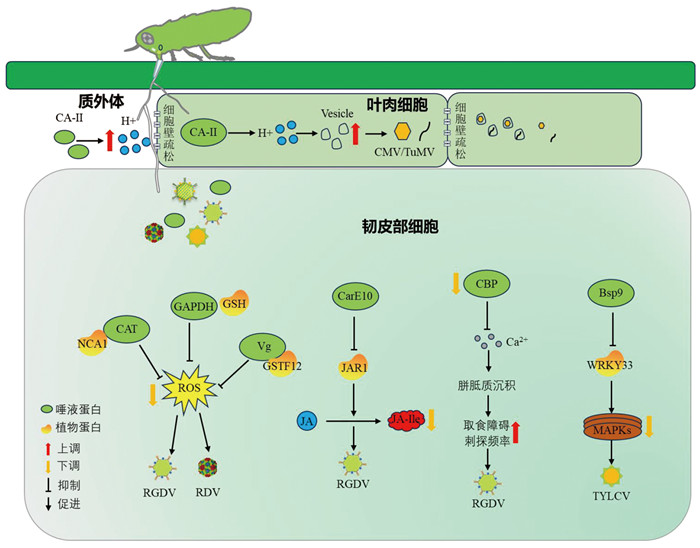
|
蚜虫唾液CA-Ⅱ通过增强叶肉细胞质外体酸化,加快植物囊泡运输,促进CMV和TuMV的胞间转运。叶蝉唾液效应子CAT、Vg通过分别与寄主韧皮部NCA1、GSTF12互作抑制水稻H2O2的积累,GAPDH通过还原水稻H2O2降低其积累量,这些过程都抑制水稻H2O2爆发;CarE10通过抑制JA-Ile的合成抑制水稻抗虫反应,促进昆虫取食,进而促进了RGDV或RDV的传播。叶蝉CBP可结合Ca2+,降低游离的Ca2+浓度,延缓胼胝质的积累,而RGDV通过抑制CBP的表达,诱导水稻产生高浓度Ca2+,促进韧皮部胼胝质沉积,从而增加叶蝉刺探频率,促进RGDV传播。TYLCV诱导烟粉虱唾液蛋白Bsp9与抗性反应相关转录因子WRKY33互作,抑制MAPK信号途径的抗性反应,促进昆虫取食和自身传播。 图 2 唾液蛋白调控植物防御反应促进病毒传播的示意图 |
昆虫唾液蛋白与植物互作调控昆虫取食和病毒传播的研究已成为热点。虽然蚜虫、烟粉虱、飞虱和叶蝉等的唾液蛋白参与调控寄主植物免疫反应的研究已取得一定的进展[10, 67-68],但是仍有许多唾液蛋白调控植物防御反应的作用机制是模糊不清的,仍有待进一步的阐述。
目前,利用抗虫基因进行遗传改良和品种选育仍是农业害虫防治的重要策略,但是R基因的鉴定和克隆将是未来相关研究面临的巨大挑战。近年来,仅有少数几种对刺吸式昆虫具有抗性作用的NLR受体(Mi、Vat、BPH14等)被鉴定[58-59],对于触发NLR受体、激活ETI免疫反应的无毒效应子更是知之甚少。寄主植物如何识别昆虫并部署和调节抗性仍在很大程度上是未知的。昆虫唾液蛋白作为昆虫重要的效应子形式抑制植物防御反应、促进昆虫取食,也可被NLR受体识别激活ETI [46]。昆虫唾液效应子的研究将对NLR抗性基因的发掘和鉴定具有极大的促进作用。发掘、鉴定识别昆虫唾液效应子的R基因将是未来防治植食性昆虫的重要研究方向。
植物虫媒病毒的侵染调控昆虫唾液效应蛋白的合成、分泌和释放,以唾液蛋白为出发点,深入探究病毒-昆虫-植物的复杂相互作用关系,将是揭示植物病毒水平传播机制的重要手段。随着对唾液蛋白调控机制的不断深入解析,唾液蛋白将是以阻断病毒跨界传播为病害防控策略的重要靶标。
| [1] |
Sarmento RA, Lemos F, Bleeker PM, et al. A herbivore that manipulates plant defence. Ecol Lett, 2011, 14: 229-36. DOI:10.1111/j.1461-0248.2010.01575.x |
| [2] |
van Bel AJ, Will T. Functional evaluation of proteins in watery and gel saliva of aphids. Front Plant Sci, 2016, 7: 1840. |
| [3] |
He WB, Li J, L iu, S S. Differential profiles of direct and indirect modification of vector feeding behaviour by a plant virus. Sci Rep, 2015, 5: 7682. DOI:10.1038/srep07682 |
| [4] |
Stafford CA, Walker GP, Ullman DE. Infection with a plant virus modifies vector feeding behavior. Proc Natl Acad Sci U S A, 2011, 108: 9350-5. DOI:10.1073/pnas.1100773108 |
| [5] |
Huang HJ, Liu CW, Cai YF, et al. A salivary sheath protein essential for the interaction of the brown planthopper with rice plants. Insect Biochem Mol Biol, 2015, 66: 77-87. DOI:10.1016/j.ibmb.2015.10.007 |
| [6] |
Huang HJ, Liu CW, Xu HJ, et al. Mucin-like protein, a saliva component involved in brown planthopper virulence and host adaptation. J Insect Physiol, 2017, 98: 223-30. DOI:10.1016/j.jinsphys.2017.01.012 |
| [7] |
Kettles GJ, Kaloshian I. The potato aphid salivary effector Me47 is a glutathione-S-transferase involved in modifying plant responses to aphid infestation. Front Plant Sci, 2016, 7: 1142. |
| [8] |
Furch ACU, van Bel AJE, Will T. Aphid salivary proteases are capable of degrading sieve-tube proteins. J Exp Bot, 2015, 66: 533-9. DOI:10.1093/jxb/eru487 |
| [9] |
Will T, Tjallingii WF, Thonnessen A, et al. Molecular sabotage of plant defense by aphid saliva. Proc Natl Acad Sci U S A, 2007, 104: 10536-41. DOI:10.1073/pnas.0703535104 |
| [10] |
Naalden D, van Kleeff PJM, Dangol S, et al. Spotlight on the roles of whitefly effectors in insect-plant interactions. Front Plant Sci, 2021, 12: 661141. DOI:10.3389/fpls.2021.661141 |
| [11] |
Fu J, Shi Y, Wang L, et al. Planthopper-secreted salivary disulfide isomerase activates immune responses in plants. Front Plant Sci, 2020, 11: 622513. |
| [12] |
Zeng J, Ye W, Hu W, et al. The N-terminal subunit of vitellogenin in planthopper eggs and saliva acts as a reliable elicitor that induces defenses in rice. New Phytol, 2023, 238: 1230-44. DOI:10.1111/nph.18791 |
| [13] |
Huang J, Zhang N, Shan J, et al. Salivary protein 1 of brown planthopper is required for survival and induces immunity response in plants. Front Plant Sci, 2020, 11: 571280. DOI:10.3389/fpls.2020.571280 |
| [14] |
Chaudhary R, Atamian HS, Shen Z, et al. Potato aphid salivary proteome: enhanced salivation using resorcinol and identification of aphid phosphoproteins. J Proteome Res, 2015, 14: 1762-78. DOI:10.1021/pr501128k |
| [15] |
Huang HJ, Yan XT, Wei ZY, et al. Identification of riptortus pedestris salivary proteins and their roles in inducing plant defenses. Biology, 2021, 10: 753. DOI:10.3390/biology10080753 |
| [16] |
Liu X, Zhou H, Zhao J, et al. Identification of the secreted watery saliva proteins of the rice brown planthopper, Nilaparvata lugens (Stal) by transcriptome and Shotgun LC-MS/MS approach. J Insect Physiol, 2016, 89: 60-9. DOI:10.1016/j.jinsphys.2016.04.002 |
| [17] |
Vincent TR, Avramova M, Canham J, et al. Interplay of plasma membrane and vacuolar ion channels, together with BAK1, eicits rapid cytosolic calcium elevations in Arabidopsis during aphid feeding. Plant Cell, 2017, 29: 1460-79. DOI:10.1105/tpc.17.00136 |
| [18] |
Pieterse CM, Van der Does D, Zamioudis C, et al. Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol, 2012, 28: 489-521. DOI:10.1146/annurev-cellbio-092910-154055 |
| [19] |
Cui N, Lu H, Wang T, et al. Armet, an aphid effector protein, induces pathogen resistance in plants by promoting the accumulation of salicylic acid. Philos Trans R Soc B Biol Sci, 2019, 374. |
| [20] |
Wang W, Dai H, Zhang Y, et al. Armet is an effector protein mediating aphid-plant interactions. FASEB J, 2015, 29: 2032-45. DOI:10.1096/fj.14-266023 |
| [21] |
Wu J, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Annu Rev Genet, 2010, 44: 1-24. DOI:10.1146/annurev-genet-102209-163500 |
| [22] |
Elzinga DA, De Vos M, Jander G. Suppression of plant defenses by a Myzus persicae (green peach aphid) salivary effector protein. Mol Plant Microb Interact, 2014, 27: 747-56. DOI:10.1094/MPMI-01-14-0018-R |
| [23] |
Naessens E, Dubreuil G, Giordanengo P, et al. A secreted MIF cytokine enables aphid feeding and represses plant immune responses. Curr Biol, 2015, 25: 1898-903. DOI:10.1016/j.cub.2015.05.047 |
| [24] |
Ye W, Yu H, Jian Y, et al. A salivary EF-hand calcium-binding protein of the brown planthopper Nilaparvata lugens functions as an effector for defense responses in rice. Sci Rep, 2017, 7: 40498. DOI:10.1038/srep40498 |
| [25] |
Fu J, Shi Y, Wang L, et al. Planthopper-secreted salivary calmodulin acts as an effector for defense responses in rice. Front Plant Sci, 2022, 13: 841378. DOI:10.3389/fpls.2022.841378 |
| [26] |
Tian T, Ji R, Fu J, et al. A salivary calcium-binding protein from Laodelphax striatellus acts as an effector that suppresses defense in rice. Pest Manag Sci, 2021, 77: 2272-81. DOI:10.1002/ps.6252 |
| [27] |
Huang HJ, Cui JR, Xia X, et al. Salivary DNase Ⅱ from Laodelphax striatellus acts as an effector that suppresses plant defence. New Phytol, 2019, 224: 860-74. DOI:10.1111/nph.15792 |
| [28] |
Zhang H, Chi Y, Chen S, et al. Scavenging H2O2 of plant host by saliva catalase of leafhopper vector benefits viral transmission. New Phytol, 2024, 243: 2368-84. DOI:10.1111/nph.19988 |
| [29] |
Wang Y, Lu C, Guo S, et al. Leafhopper salivary vitellogenin mediates virus transmission to plant phloem. Nat Commun, 2024, 15: 3. |
| [30] |
Wang X, Wu H, Yu Z, et al. Plant viruses exploit insect salivary GAPDH to modulate plant defenses. Nat Commun, 2024, 15: 6918. DOI:10.1038/s41467-024-51369-8 |
| [31] |
Liu H, Wang C, Qiu CL, et al. A salivary odorant-binding protein mediates Nilaparvata lugens feeding and host plant phytohormone suppression. In J Mol Sci, 2021, 22: 4988. DOI:10.3390/ijms22094988 |
| [32] |
Huang HJ, Wang YZ, Li LL, et al. Planthopper salivary sheath protein LsSP1 contributes to manipulation of rice plant defenses. Nat Commun, 2023, 14: 737. DOI:10.1038/s41467-023-36403-5 |
| [33] |
Chi Y, Zhang H, Chen S, et al. Leafhopper salivary carboxylesterase suppresses JA-Ile synthesis to facilitate initial arbovirus transmission in rice phloem. Plant Commun, 2024, 5: 100939. DOI:10.1016/j.xplc.2024.100939 |
| [34] |
Koornneef A, Pieterse CM. Cross talk in defense signaling. Plant Physiol, 2008, 146: 839-44. DOI:10.1104/pp.107.112029 |
| [35] |
Zarate SI, Kempema LA, Walling LL. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol, 2007, 143: 866-75. DOI:10.1104/pp.106.090035 |
| [36] |
Xu HX, Qian LX, Wang XW, et al. A salivary effector enables whitefly to feed on host plants by eliciting salicylic acid-signaling pathway. Proc Natl Acad Sci U S A, 2019, 116: 490-5. DOI:10.1073/pnas.1714990116 |
| [37] |
Carolan JC, Fitzroy CIJ, Ashton PD, et al. The secreted salivary proteome of the pea aphid Acyrthosiphon pisum characterised by mass spectrometry. Proteomics, 2009, 9: 2457-67. DOI:10.1002/pmic.200800692 |
| [38] |
Knoblauch M, Peters W, Ehlers K, et al. Reversible calcium-regulated stopcocks in legume sieve tubes. Plant Cell Environ, 2001, 13: 1221-30. |
| [39] |
Walling LL. The myriad plant responses to herbivores. J Plant Growth Regul, 2000, 19: 195-216. DOI:10.1007/s003440000026 |
| [40] |
Verma DPS, Hong Z. Plant callose synthase complexes. Plant Mol Biol, 2001, 47: 693-701. DOI:10.1023/A:1013679111111 |
| [41] |
Will T, van Bel AJE. Physical and chemical interactions between aphids and plants. J Exp Bot, 2006, 57: 729-37. DOI:10.1093/jxb/erj089 |
| [42] |
Wu W, Yi G, Lv X, et al. A leafhopper saliva protein mediates horizontal transmission of viral pathogens from insect vectors into rice phloem. Commun Biol, 2022, 5: 204. DOI:10.1038/s42003-022-03160-y |
| [43] |
Cui H, Tsuda K, Parker JE. Effector-triggered immunity: from pathogen perception to robust defense. Annu Rev Plant Biol, 2015, 66: 487-511. DOI:10.1146/annurev-arplant-050213-040012 |
| [44] |
Kaloshian I. Gene-for-gene disease resistance: bridging insect pest and pathogen defense. J Chem Ecol, 2004, 30: 2419-38. DOI:10.1007/s10886-004-7943-1 |
| [45] |
Du B, Zhang W, Liu B, et al. Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc Natl Acad Sci U S A, 2009, 106: 22163-8. DOI:10.1073/pnas.0912139106 |
| [46] |
Guo J, Wang H, Guan W, et al. A tripartite rheostat controls self-regulated host plant resistance to insects. Nature, 2023, 618: 799-807. DOI:10.1038/s41586-023-06197-z |
| [47] |
Webster CG, Pichon E, van Munster M, et al. Identification of plant virus receptor candidates in the stylets of their aphid vectors. J Virol, 2018, 92: e00432-18. |
| [48] |
Ng JCK, Perry KL. Transmission of plant viruses by aphid vectors. Mol Plant Pathol, 2004, 5: 505-11. |
| [49] |
Zhou JS, Drucker M, Ng JCK. Direct and indirect influences of virus-insect vector-plant interactions on non-circulative, semi-persistent virus transmission. Curr Opin Virol, 2018, 33: 129-36. |
| [50] |
Guo H, Zhang Y, Li B, et al. Salivary carbonic anhydrase Ⅱ in winged aphid morph facilitates plant infection by viruses. Proc Natl Acad Sci U S A, 2023, 120: e2222040120. |
| [51] |
Blanc S, Michalakis Y. Manipulation of hosts and vectors by plant viruses and impact of the environment. Curr Opin Insect Sci, 2016, 16: 36-43. |
| [52] |
Mauck KE. Variation in virus effects on host plant phenotypes and insect vector behavior: what can it teach us about virus evolution?. Curr Opin Virol, 2016, 21: 114-23. |
| [53] |
Navarro JA, Sanchez-Navarro JA, Pallas V. Key checkpoints in the movement of plant viruses through the host. Adv Virus Res, 2019, 104: 1-64. |
| [54] |
Hernández JA, Gullner G, Clemente-Moreno MJ, et al. Oxidative stress and antioxidative responses in plant-virus interactions. Physiol Mol Plant Pathol, 2016, 94: 134-48. |
| [55] |
Hyodo K, Hashimoto K, Kuchitsu K, et al. Harnessing host ROS-generating machinery for the robust genome replication of a plant RNA virus. Proc Natl Acad Sci U S A, 2017, 114: E1282-90. |
| [56] |
Fátyol K, Fekete KA, Ludman M, et al. Double-stranded-RNA-binding protein 2 participates in antiviral defense. J Virol, 2020, 94: e00017-20. |
| [57] |
Tungadi T, Watt LG, Groen SC, et al. Infection of Arabidopsis by cucumber mosaic virus triggers jasmonate-dependent resistance to aphids that relies partly on the pattern-triggered immunity factor BAK1. Mol Plant Pathol, 2021, 22: 1082-91. |
| [58] |
Snoeck S, Guayazán-Palacios N, Steinbrenner AD. Molecular tug-of-war: plant immune recognition of herbivory. Plant Cell, 2022, 34: 1497-513. |
| [59] |
Boissot N. NLRs are highly relevant resistance genes for aphid pests. Curr Opin Insect Sci, 2023, 56: 101008. |
| [60] |
Vincent TR, Avramova M, Canham J, et al. Interplay of plasma membrane and vacuolar ion channels, together with BAK1, elicits rapid cytosolic calcium elevations in Arabidopsis during aphid feeding. Plant Cell, 2017, 29: 1460-79. |
| [61] |
Carr JP, Murphy AM, Tungadi T, et al. Plant defense signals: players and pawns in plant-virus-vector interactions. Plant Sci, 2019, 279: 87-95. |
| [62] |
Hogenhout SA, Ammar El-D, Whitfield AE, et al. Insect vector interactions with persistently transmitted viruses. Annu Rev Phytopathol, 2008, 46: 327-59. |
| [63] |
Lei W, Li P, Han Y, et al. EPG recordings reveal differential feeding behaviors in Sogatella furcifera in response to plant virus infection and transmission success. Sci Rep, 2016, 6: 30240. |
| [64] |
Wang Q, Li J, Dang C, et al. Rice dwarf virus infection alters green rice leafhopper host preference and feeding behavior. PLoS One, 2018, 13: e0203364. |
| [65] |
He YZ, Wang YM, Yin TY, et al. A plant DNA virus replicates in the salivary glands of its insect vector via recruitment of host DNA synthesis machinery. Proc Natl Acad Sci U S A, 2020, 117: 16928-37. |
| [66] |
Wang N, Zhao P, Ma Y, et al. A whitefly effector Bsp9 targets host immunity regulator WRKY33 to promote performance. Philos Trans R Soc B Biol Sci, 2019, 374. |
| [67] |
Huang HJ, Zhang CX, Hong XY. How does saliva function in planthopper-host interactions?. Arch Insect Biochem, 2019, 100: e21537. |
| [68] |
张艳静, 李丹阳, 郭慧娟, 等. 蚜虫传播非持久性病毒的取食行为调控机制. 植物保护学报, 2020, 47: 949-61. |
 2025, Vol. 37
2025, Vol. 37 

 魏太云,福建农林大学植物保护学院研究员。2003年毕业于福建农林大学植物病理学专业,获得农学博士专业;2003—2009年分别在日本国立农业研究中心和加拿大农业与农业食品部从事博士后研究。2010年任职于福建农林大学,聚焦媒介昆虫传播植物病原包括水稻病毒、水稻植原体和柑橘黄龙病菌的致灾机制与调控策略研究,在Nature Microbiology、PNAS、Autophagy、Nature Communications等刊物发表100余篇研究论文。主持国家重点研发计划课题、基金委重点项目、重点国际合作和创新发展联合基金重点项目等,作为第一完成人获教育部自然科学奖二等奖和福建省自然科学奖二等奖。入选教育部长江学者特聘教授,国家杰出青年科学基金获得者,现任国际病毒分类委员会(ICTV)成员,Molecular Plant-Microbe Interactions、Virology Journal及Phytopathology Research编委
魏太云,福建农林大学植物保护学院研究员。2003年毕业于福建农林大学植物病理学专业,获得农学博士专业;2003—2009年分别在日本国立农业研究中心和加拿大农业与农业食品部从事博士后研究。2010年任职于福建农林大学,聚焦媒介昆虫传播植物病原包括水稻病毒、水稻植原体和柑橘黄龙病菌的致灾机制与调控策略研究,在Nature Microbiology、PNAS、Autophagy、Nature Communications等刊物发表100余篇研究论文。主持国家重点研发计划课题、基金委重点项目、重点国际合作和创新发展联合基金重点项目等,作为第一完成人获教育部自然科学奖二等奖和福建省自然科学奖二等奖。入选教育部长江学者特聘教授,国家杰出青年科学基金获得者,现任国际病毒分类委员会(ICTV)成员,Molecular Plant-Microbe Interactions、Virology Journal及Phytopathology Research编委