随着全球人口激增和环境恶化的加剧,植物病害的发生愈发频繁,给农业生产带来了前所未有的挑战。长期以来,农业生产高度依赖化学农药来控制植物病害,但化学农药的过度使用不仅成本高昂,还可能对环境和人类健康造成负面影响。因此,深入研究并利用植物自身的免疫系统,成为开发绿色防控生物技术的关键[1]。
植物病原菌种类繁多,根据其生活方式和与宿主的关系,大致可分为死体营养型、活体营养型和半活体营养型。死体营养型病原菌依赖死亡的宿主细胞获取养分,而活体营养型则直接从活细胞中汲取营养。半活体营养型病原菌的寄生过程则更为复杂,它们最初以活体营养方式侵入,从活体宿主细胞获取营养,随后转向死体营养阶段[2]。
植物免疫系统是植物在自然界中抵御外界病原菌侵染的重要防线。针对不同类型的病原菌,植物会采取不同的防御策略。在与病原菌协同进化的过程中,植物演化出了两层典型免疫系统来抵御病原菌攻击。第一层免疫系统是保守分子模式触发的免疫(pattern-triggered immunity, PTI),它通过细胞质膜上的模式识别受体(pattern recognition receptor, PRR)识别病原菌相关分子模式(pathogen-associated molecular pattern, PAMP)或损伤相关分子模式(damage- associated molecular pattern, DAMP),从而触发免疫。为了成功地侵染宿主,病原菌分泌效应子(effector)来干扰植物的PTI,触发植物的感病性(effector-triggered susceptibility, ETS)。而植物则可进化出胞内的NLR (nucleotide-binding, leucine-rich repeat proteins)受体蛋白直接或间接地识别病原菌的效应子,激活第二层免疫,即效应子触发的免疫(effector-triggered immunity, ETI),从而激发更为强烈和持久的免疫反应[3, 4]。
植物激素在协调植物免疫方面发挥着至关重要的作用[5-7]。其中茉莉酸(jasmonic acid, JA)及其信号途径在植物与病原菌互作中发挥重要作用。研究表明,病原菌入侵后会干扰宿主茉莉酸的合成、代谢和信号途径,茉莉酸途径在植物应对不同病原菌的免疫中发挥不同的作用[2, 8]。近年来,茉莉酸途径在调控植物抗性方面的研究取得了一系列重要进展。本文系统梳理了茉莉酸的生物合成、代谢、信号通路,以及其在植物与不同类型病原菌互作中的作用和植物免疫中的功能,并对未来的研究方向和应用进行了展望。深入解析茉莉酸途径在植物免疫信号转导中的核心作用及其分子机制,有望为农业生产中的病害防控提供新的视角和方法,进而对减少化学农药使用、提高作物抗病性、保障农作物的产量和质量,以及促进环境的可持续性具有重要意义。
1 茉莉酸的生物合成及代谢茉莉酸是一类重要的脂质植物激素,涵盖茉莉酸及其衍生的环戊烷酮类化合物[9, 10]。随着分子生物学的飞速发展和科学家们的不懈探索,对茉莉酸的生物合成和代谢过程有了深刻的认识,并建立了一个详尽的动态模型(图 1)。
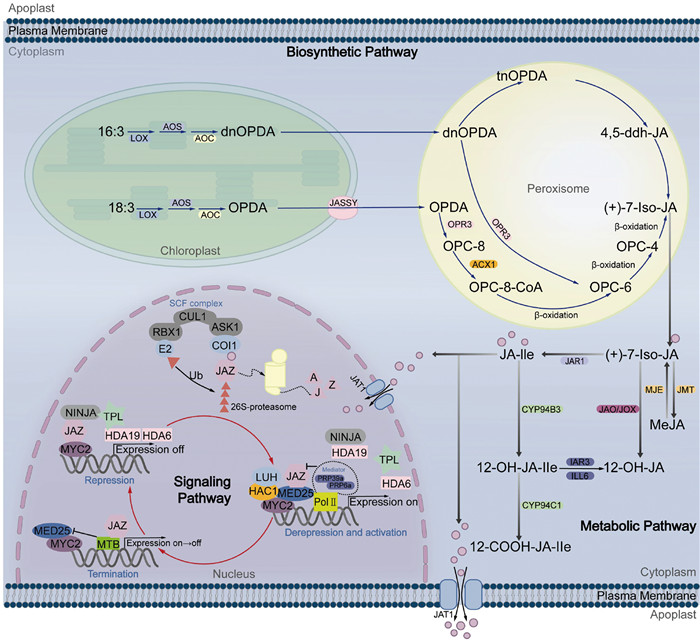
|
图 1 茉莉酸的生物合成、代谢和信号途径模式图 |
茉莉酸的生物合成过程起始于叶绿体内,通过十八碳烷或者十六碳烷脂肪酸途径进行[10]。在这个过程中,JA的前体——不饱和脂肪酸(18:3和16:3)从叶绿体膜上释放,经历13-脂氧合酶(13-lipoxygenase, 13-LOX)、丙二烯氧化物合酶(allene oxide synthase, AOS)和丙二烯氧化物环化酶(allene oxide cyclase, AOC)的作用,逐步被转化为12-氧代-植物二烯酸(12-oxo-phytodienoic acid, 12-OPDA)及其脱氧甲基形式(deoxymethylated vegetable dienic acid, dn-OPDA)[11]。这些中间产物随后被运输出叶绿体,进入过氧化物酶体[12]。最近研究揭示叶绿体外膜上的Bet-v1类通道蛋白JASSY对于OPDA从叶绿体中输出至关重要[13]。在过氧化物酶体中,OPDA和dn-OPDA经OPDA还原酶3 (12-oxophytodienoate reductase 3, OPR3)还原和一系列复杂的氧化反应转化为(+)-7-iso-JA,从而完成JA的生物合成[14]。同时,对拟南芥(Arabidopsis thaliana) opr3突变体的研究揭示了一条不依赖OPR3的JA生物合成新途径,其中OPDA在没有OPR3的情况下可通过β-氧化产生4, 5-二脱氢茉莉酮酸酯(4, 5-didehydrojasmonate, 4, 5-ddh-JA),作为JA及其活性形式JA-Ile的直接前体[15]。
(+)-7-iso-JA在过氧化酶体中生成后,被转运至细胞质并经过进一步修饰,形成多样的代谢产物[16]。JA的主要活性形式为氨基酸结合物,其中异亮氨酸(L-isoleucine, L-Ile)是与JA结合的主要氨基酸。JA在依赖ATP的腺苷酸形成酶JAR1 (jasmonic acid resistant 1)的催化下与异亮氨酸结合,形成具有生物活性的JA-Ile[17]。JA-Ile随后进入细胞核,启动茉莉酸信号转导途径。研究表明,拟南芥中ABC转运蛋白JAT1/ABCG16介导JA跨细胞质膜的外运以及JA-Ile跨核膜的内运[18]。此外,JA可通过多样的代谢途径进一步转化,包括通过甲基化作用形成茉莉酸甲酯(methyl jasmonate, MeJA)、通过形成葡萄糖酯结合JA、脱羧反应生成顺式茉莉酮(cis-jasmone),以及羟基化反应产生12-OH-JA,展现了其在植物体内的复杂代谢网络[19]。正是这些复杂的合成和代谢过程共同维持了植物体内茉莉酸的平衡。
2 茉莉酸的信号转导途径茉莉酸信号转导途径由多个相互关联的功能模块组成。在该途径中,茉莉酸的受体COI1 (coronatine-insensitive 1)与JAZs (jasmonate-ZIM domain)蛋白相互作用,共同调控信号的激活与抑制。在没有JA-Ile的情况下,JAZ蛋白抑制下游转录因子如MYC2的转录活性,维持信号途径的抑制状态。当JA-Ile存在时,JAZ蛋白被泛素化降解,从而释放MYC2等下游转录因子,激活茉莉酸响应基因的表达。同时,MYC2通过激活bHLH转录因子MTBs (MYC2-targeted bHLHs)使其抑制MYC2的转录活性,最终实现JA信号途径的适时终止[20](图 1)。
在正常生长条件下,植物体内的JA-IIe水平较低,JA信号途径保持在非激活状态[21, 22]。JAZ蛋白通过与MYC2等转录因子结合,并募集一系列转录抑制因子,如NINJA (novel interactor of JAZ)和TPL (topless)等,共同抑制茉莉酸响应基因的转录。在拟南芥中存在13个JAZ蛋白,它们属于植物特异性TIFY家族,包含带有核心基序TIF[F/Y]XG的ZIM结构域,以及相对保守的N端NT结构域和高度保守的C端Jas结构域[23, 24]。ZIM结构域介导JAZ蛋白与NINJA蛋白相互作用,后者募集TPL形成JAZ-NINJA-TPL抑制复合体[25]。研究表明,NINJA-TPL的抑制活性依赖乙酰化修饰。组蛋白去乙酰化酶HDA6 (histone deacetylase 6)介导的TPL去乙酰化减弱了TPL-NINJA的作用和TPL对MYC2靶启动子的募集,从而增强了JA响应基因的表达。反之,乙酰化TPL抑制JA响应基因的表达[26]。JAZ蛋白数量众多且功能部分冗余,为JA信号途径的复杂性和特异性提供了生物基础,其中JAZ3/ 4/6/9/10/11/12在植物对病虫害的响应中具有重要作用[27]。
当植物遭受病原菌入侵等胁迫时,JA-IIe迅速合成。这种活性形式的茉莉酸能够精准地嵌入由茉莉酸受体F-box蛋白COI1的亮氨酸重复序列(LRR)构成的结合口袋中,而JAZ蛋白通过其Jas结构域与COI1相互作用,像盖子一样覆盖并封闭这个结合口袋,确保JA-Ile稳定地结合于其中[28, 29]。COI1与接头蛋白SKP1和Cullin骨架蛋白CULLIN1形成SCFCOI1E3泛素连接酶复合体,该复合体负责将JAZ蛋白泛素化,随后JAZ蛋白被26S蛋白酶体降解,从而激活JA响应基因的表达[30]。JAZ蛋白的稳定性不仅受到JA-IIe-COI1复合体的调控,还可能被其他蛋白调控。例如,在番茄(Solanum lycopersicum)中,E3泛素连接酶SlPUB22通过泛素化降解SlJAZ4激活JA信号途径[31]。在苹果(Malus domestica)中,E3泛素连接酶MdSINA11通过促进MdJAZ2的泛素化降解,参与调控花青素的积累[32]。
JA信号途径的关键转录因子,如MYC2/3/4/5,属bHLH家族,它们通过与G-box (CACGTG)及G-box相关六聚体结合,调控JA响应基因的表达。在拟南芥中,MYCs转录因子家族通过差异化调节,精细控制两组JA响应基因的表达。这些基因分别承担着植物对机械损伤和病原菌攻击的不同应答任务[33-36]。除了MYCs,JA信号途径的调控还涉及其他多个转录因子家族,包括R2R3 MYB家族、WRKY家族、NAC家族和ERF家族等,它们共同构成了一个复杂的转录调控网络[37-41]。值得注意的是,近期研究揭示了核因子NF-YB2/YB3也可以调控JA途径。NF-YB2/YB3通过招募组蛋白去甲基化酶REF6 (relative of early flowing 6),降低JA响应基因位点的H3K27me3修饰水平,并与JAZ蛋白互作,促进JAZ蛋白降解,从而激活JA相关基因的表达[42]。
转录中介体(mediator)是由多个亚基组成的转录辅助因子,在调控JA信号途径中发挥重要作用。如MED10b (mediator of RNA polymerase Ⅱ transcription subunit 10b)和MED7也与JAZ蛋白互作,共同发挥转录抑制因子的作用[43]。MED25与多种JA信号途径相关遗传和表观因子互作,实现JA信号途径中遗传和表观因子的有序整合[44, 45],MED25可以与COI1和MYC2互作,促进JAZ蛋白降解并将RNA聚合酶Ⅱ (RNA polymerase Ⅱ, Pol Ⅱ)招募到MYC2靶启动子区,用于转录起始前复合体(promote pre-initiation complex, PIC)的组装[46]。同时,MED25和转录共激活子LUH (leunig homolog)通过招募组蛋白乙酰转移酶HAC1 (histone acetyltransferase of the CBP family 1)来调节MYC2靶启动子区H3K9ac修饰水平,促进下游靶基因表达[47, 48]。MED25还能招募剪切因子PRP39a/40a (pre-mRNA-processing protein 39a/40a)通过调控JAZ剪切变体的水平严格控制JA信号激活的强度[49]。
虽然JA信号途径的激活有利于植物应对生物和非生物胁迫,但如果过度激活反而会影响植物的生长发育。因此,JA信号途径激活强度控制和适时终止对植物适应复杂环境至关重要。除了调控JAZ剪切变体水平控制信号强度外,研究还发现MYC2会激活MTBs表达。MTBs蛋白一方面阻止MYC2-MED25复合体的形成,另一方面与MYC2竞争结合靶基因的启动子,MYC2与MTBs形成一个高度有序的负反馈调控回路,实现茉莉酸信号的适时终止[20]。这些发现进一步加深了我们对茉莉酸信号转导途径复杂性的理解,并为揭示植物如何精细调控免疫提供了新的视角。
3 茉莉酸途径在植物与病原菌互作中的作用茉莉酸途径在植物防御体系中扮演着关键角色,但其功能在面对不同类型的病原菌时表现出差异性。传统观点认为茉莉酸在植物抵御死体营养型病原菌和植食性害虫中发挥重要作用,而在应对活体和半活体营养型病原菌时反而被病原菌所利用[50]。近年来,随着研究的不断深入,人们对茉莉酸途径的认识不断深化,揭示了其在植物免疫中的复杂性和多样性(图 2)。
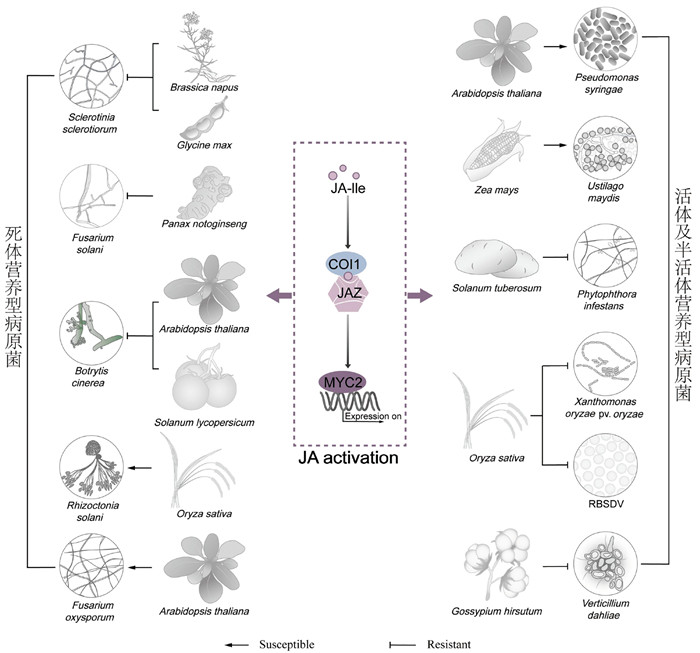
|
图 2 茉莉酸途径在植物与病原菌互作中的作用 |
JA含量提高可激活JA信号途径和防卫相关基因的表达,从而提高植物对死体营养型病原菌的抗性。例如,MeJA处理能显著增强油菜(Brassica napus)和大豆(Glycine max)对核盘菌(Sclerotinia sclerotiorum)的抗性[51, 52]以及三七(Panax notoginseng)对镰刀菌(Fusarium solani)[53, 54]和月季(Rosa chinensis)对灰霉(Botrytis cinerea)的抗性[55]。此外,机械损伤预处理拟南芥幼苗可使其JA含量升高,从而增强对死体营养型病原菌的抗性[56]。系统素(systemin, SYS)是植物中发现较早的多肽信号分子之一,在番茄中SYS可通过诱导JA信号途径,激活防卫相关基因表达,增强其对灰霉的抗性[57]。对番茄opr3突变体研究发现,在抵御死体营养型病原菌的过程中,OPDA可以通过诱导胼胝质积累提高植物防御能力[58]。此外,在拟南芥中,JA生物合成关键酶酰基辅酶A氧化酶(ACX)的缺失导致JA水平下降,进而使突变体对灰霉的敏感性显著增加[59, 60]。
植物可通过调节JA的活性和非活性形式或代谢响应病原菌的侵染。在拟南芥中,CYP94 (cytochrome P450 94)家族的成员通过ω-氧化作用使JA-Ile失活,其中AtCYP94B1和AtCYP94B3负责将具有生物活性的JA-Ile转化为活性较低的12-OH-JA-Ile,而AtCYP94C1进一步将12-OH-JA-Ile转化为完全失去活性的12-COOH-JA-Ile。这种代谢调节在番茄中也有报道。敲除SlCYP94C1基因后,成熟果实中JA-Ile含量显著增加,从而有效提高了果实对死体营养型病原菌的抗性[61]。进一步的研究发现,在灰霉侵染过程中,F-box蛋白BFP1 (Botrytis cinerea-induced F-box protein 1)的表达水平上升,该蛋白通过泛素化茉莉酸代谢关键酶JAO/JOXs (jasmonic acid oxidases/jasmonate-induced oxygenases),抑制JA的分解代谢,从而在植物体内积累高水平的JA,增强植物对灰霉的抗性[62]。
茉莉酸信号转导途径在植物抵御死体营养型病原菌中发挥重要作用[63]。在番茄中,COI1同源基因JAI1的突变导致植株更易受灰霉侵染,并且MYC2-RNAi植株在遭受灰霉侵染后,病斑面积明显大于野生型植株[64]。BOS1编码的R2R3 MYB转录因子的表达在灰霉侵染时上调,而在coi1突变体中这一过程受到显著抑制[65]。在拟南芥中,转录因子WRKY75基因的过表达增强了JA途径介导的植物对灰霉和链格孢菌(Alternaria Nees)的抗性[66]。同样,在水稻(Oryza sativa)中,转录因子OsWRKY28基因的缺失导致植物对立枯丝核菌(Rhizoctonia solani)的抗性减弱[67]。这些研究进一步揭示了JA信号转导途径在植物免疫中的重要作用。
然而,死体营养型病原菌亦能巧妙地操纵植物的JA途径以促进其侵染。例如,coi1突变体对尖孢镰刀菌(Fusarium oxysporum)表现出更强的抗性,这暗示了JA途径在病原菌侵染中的双刃剑作用。研究进一步发现Fo5176株系可通过其效应子SIX4激活植物的JA途径,以此促进其对植物的侵染[68, 69]。此外,链格孢菌分泌的毒素TeA能够诱导高水平的JA积累及其响应基因的表达,从而推动叶片病害的发展[70]。可见,死体营养型病原菌也可以通过激活茉莉酸途径来干扰植物免疫,展现了植物与病原菌之间复杂的相互作用。
3.2 茉莉酸在植物与活体及半活体营养型病原菌互作中的作用学界普遍认为水杨酸(salicylic acid, SA)途径在植物抵御活体和半活体营养型病原菌中发挥核心作用。由于SA和JA途径之间存在拮抗关系,人们通常认为JA途径在植物抵御活体和半活体营养型病原菌中起负调节作用。尽管有大量证据支持这一观点,但JA途径在植物抵御某些活体和半活体营养型病原菌中也发挥重要功能,表明JA途径在植物与病原菌互作中功能的多样性和复杂性。
活体营养型病原菌可通过多种方式激活JA途径,促进其侵染。病原菌可以通过合成茉莉酸类似物激活JA途径,干扰植物免疫[71]。例如,丁香假单胞菌(Pseudomonas syringae)能够合成冠菌素(COR),这是一种茉莉酸类似物,它能够与茉莉酸受体COI1结合,导致JAZ蛋白降解,激活JA途径[72],同时抑制SA途径,促进丁香假单胞菌侵染[73-75]。此外,病原菌也能利用自身分泌的效应子激活JA生物合成和信号转导途径,促进其侵染。如丁香假单胞菌效应子AvrB能通过与MPK4及分子伴侣HSP90 (heat shock protein 90)相互作用,促进MPK4的磷酸化,激活JA途径[76]。同时,AvrB还能与RIN4 (RPM1-interacting protein 4)互作,促进COI1与JAZ蛋白的互作,进而激活JA途径[77]。效应子HopZ1a和HopX1也可通过不同机制调控JAZ蛋白,激活JA途径。HopZ1a通过乙酰化JAZ蛋白促进其降解[78],而HopX1则通过其半胱氨酸蛋白酶活性降解JAZ蛋白[79]。效应子HopBB1通过促进转录因子TCP14 (teosinte branched 1/cincinnata/ proliferating cell factor 14)蛋白的降解,解除了TCP14对JA响应基因的抑制作用,激活JA途径。此外,HopBB1与JAZ3蛋白的互作也减弱了JAZ3对转录因子MYC2的抑制作用,从而激活JA途径[80]。能引发玉米(Zea mays)黑粉病的黑粉菌(Ustilago maydis)可利用效应子Jsi1与TPL蛋白互作,解除TPL蛋白对JA响应基因的抑制作用,促进其侵染[81]。
JA途径的激活也可提高植物对某些活体和半活体营养型病原菌的抗性。例如,外施MeJA可以显著增强马铃薯(Solanum tuberosum)对致病疫霉(Phytophthora infestans)的抗性,进一步研究发现致病疫霉效应子PiAVR3b可以干扰茉莉酸的合成,从而抑制JA途径[82]。大豆疫霉菌(Phytophthora sojae)效应子Avh94通过稳定GmJAZ1/2蛋白抑制JA途径,促进病原菌的侵染[83]。当半活体营养型病原菌稻瘟菌(Magnaporthe oryzae)和活体营养型病原菌白叶枯菌(Xanthomonas oryzae pv.oryzae)侵染时,植物可以通过增强转录因子OsEIL3 (ethylene insensitive- like 3)与OsERF040启动子结合激活JA途径,从而提高植物抗性[67]。白叶枯菌可通过抑制水稻JA合成相关基因OsAOS1表达,降低内源JA的含量,从而降低水稻对白叶枯病的抗性[84]。大丽轮枝菌(Verticillium dahliae)是一种半活体型病原菌,通过抑制棉花(Gossypium hirsutum)GhJAZ2的表达可提高JA的积累,从而增强棉花对该病原菌引起的棉花黄萎病抗性[85]。植物病毒危害作物产量和品质,严重时可导致作物绝产,而JA途径是抗病毒免疫调控网络的重要枢纽。如叶绿体蛋白cpSRP54 (chloroplast signal recognition particle 54 kDa protein)作用于JA生物合成的上游,并参与JA生物合成关键酶AOCs向类囊体膜的转运。芜菁花叶病毒(turnip mosaic virus, TuMV)在侵染烟草(Nicotiana benthamiana)的过程中通过降解cpSRP54实现对JA生物合成的抑制[86]。外源施加MeJA可以显著增强水稻对水稻黑条矮缩病毒(rice black-streaked dwarf virus, RBSDV)的抗性,并且在RBSDV感染过程中,受体样蛋白OsRLP1 (receptor-like protein 1)通过激活MAPK级联反应,进而激活JA途径介导的水稻广谱抗病毒免疫[87]。番茄黄曲叶病毒(tomato yellow leaf curl China virus, TYLCCNV)编码的βC1蛋白通过干扰MYC2同源二聚体的形成抑制JA途径下游多种抗虫相关次生代谢物质的合成代谢相关基因的表达,促进其自身侵染[88]。由此可见,JA途径在植物与病原菌的互作中扮演着复杂且多样的角色:一方面,病原菌可以通过操纵该途径来促进自身的侵染;另一方面,在某些情况下,茉莉酸信号途径的激活能增强植物的防御能力,展现了植物免疫系统的复杂性和精细调控。
4 茉莉酸途径在植物免疫信号转导中的作用机制植物细胞膜上的PRR或胞内NLR免疫识别受体蛋白被激活后,触发一系列免疫反应,包括钙离子内流(calcium influx)、活性氧(reactive oxygen species, ROS)爆发、丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)信号级联的激活,以及植物激素的合成。这些反应共同促进了全基因组范围内的大规模转录重编程,形成一个错综复杂的免疫信号网络,这些网络在PTI和ETI中都发挥重要作用[89, 90]。其中,JA途径在植物免疫的多个关键阶段都发挥至关重要的作用。
4.1 茉莉酸途径与植物免疫识别受体复合物相互作用茉莉酸途径能对植物细胞膜上的PRR或胞内NLR免疫识别受体的激活作出响应,这些受体或其复合体成员可以直接调控JA途径。在拟南芥中,受体激酶FER (feronia)是一类植物受体激酶CrRLK1L亚家族的成员,它有助于免疫受体激酶FLS2 (flagellin sensing 2)与其共受体BAK1 (brassinosteroid-insensitive 1-associated receptor kinase 1)形成复合体进而激活植物免疫[91]。FER通过磷酸化降解MYC2蛋白,从而抑制JA信号途径的激活[92]。在番茄中,Sw-5b是介导番茄斑萎病毒(tomato spotted wilt virus, TSWV)的NLR受体,其在识别病毒效应子NSm后,通过CC结构域干扰MED10b与MED7之间的相互作用,解除了MED10b-MED7-JAZ蛋白复合体对JA途径的抑制作用,进而激活植物免疫。同时,该研究还发现茄科植物中的辅助NLR (helper NLR),例如NRCs等多种NLRs,也采取类似的机制激活JA信号途径[43]。
4.2 茉莉酸途径与植物免疫信号通路协同作用钙离子的迅速内流和细胞氧化还原状态的动态变化是植物免疫系统不可或缺的组成部分,它们通过协同互作共同调控PTI和ETI的信号转导[93]。当植物感知到PAMPs或效应子时,钙离子的内流被迅速诱导,这一过程与钙通道的激活密切相关。抗病小体,即由病原菌效应子激活的NLR抗病蛋白与其他免疫组分一起形成的蛋白复合体,已被证实能够作为钙离子通道,调节钙离子的流动[94, 95]。在细胞内部,钙信号的传递受到钙结合蛋白如钙调蛋白CAM (calmodulin)和Ca2+依赖性蛋白激酶CDPK (calcium-dependent protein kinase)的调控。研究发现,钙调蛋白CAM2能促进JA合成酶LOX3和OPR3的表达,激活JA途径,从而提高植物对棉铃虫(Helicoverpa armigera)的抗性[96]。ROS的产生和信号转导与钙信号紧密相连,因为这些分子可以直接调节彼此在细胞内的浓度。PTI和ETI均能导致ROS的爆发,研究表明植物细胞受到损伤后,其DNA会释放到细胞外成为胞外自身DNA (extracellular self-DNA, esDNA)可通过JA途径诱导ROS产生,从而提高拟南芥对灰霉的抗性[97],可见JA途径对于ROS产生具有重要的贡献。
MAPK级联途径是PTI和ETI下游的一个重要组分,通常会激活JA途径,提高植物的抗性[76]。如在烟草(Nicotiana attenuata)中,敲除激酶MPK4基因会抑制JA响应基因的表达,进而减弱烟草对食草动物的防御能力[98]。这些研究表明,JA途径是植物免疫系统中一个关键调节因子,与免疫信号组分之间存在密切的相互作用和协同效应。
4.3 茉莉酸途径调控转录重编程茉莉酸途径的激活会导致剧烈的转录重编程,从而帮助植物从生长发育状态进入防御状态。此过程主要通过MYC2抑制参与细胞周期进程和光合作用的基因表达以及激活JA途径相关防卫基因表达完成[99-101]。同时,已有研究表明PTI和ETI过程中快速建立转录重编程均依赖于JA途径[90, 102]。并且,由于不同类型的植物细胞具有特异性,因此在植物快速启动有效的防卫反应过程中,JA途径所介导的转录因子WRKYs和MYBs可能在特定的细胞类型中发挥作用,如WRKYs在叶片表皮细胞中特异表达,而MYBs则在叶片维管细胞中高度表达以应对病原菌的威胁[103, 104]。这些发现凸显了茉莉酸途径在调控转录重编程中的重要贡献,以及其在植物免疫系统构建中的重要性。
4.4 茉莉酸途径与其他植物激素途径之间相互作用茉莉酸和其他植物激素之间错综复杂的相互作用凸显了植物免疫系统的复杂性[5]。深入理解这些相互作用机制,不仅有助于深化植物生物学的基础认识,还提供了通过有针对性地操纵激素途径提高作物抗性和生产力的潜在策略,这些策略的实施,有望在农业实践中实现作物健康管理和产量提升的双重目标。
在植物抵御病原菌侵染的过程中,茉莉酸和水杨酸途径起核心作用。通常情况下,这两种途径在植物体内呈现相互制约的拮抗关系[74, 105-107],即SA途径的激活往往会抑制JA途径,反之亦然。然而,在特定植物种类或不同环境条件下,它们也可能展现出相互促进的协同效应[108-110]。关于这两种途径如何实现拮抗和协同关系之间的动态转换,目前尚缺乏明确的理解。从目前的线索来看,这种转换可能与水杨酸受体NPR1 (nonexpressor of pathogenesis-related genes 1)和茉莉酸途径的关键转录因子MYC2之间的相互作用密切相关。例如,在拟南芥中,NPR1与MYC2可以发生互作,且NPR1与MYC2的互作能够破坏后者与MED25的结合,从而抑制JA途径的转录激活[111]。而在水稻中,NPR1可以解除JAZ蛋白与MYC2的结合,从而激活JA途径[112]。这些发现揭示了SA途径和JA途径在植物防御机制中的复杂性和多样性。
乙烯(ethylene, ET)在植物防御机制中也发挥重要作用,并与JA途径关系密切。它与JA途径的下游响应基因高度重叠,特别是在调节ERF家族成员方面,这使得两者在增强植物对病原菌的防御能力时通常表现为协同作用[96, 113-117]。然而,JA-ET的相互作用并不总是协同的,在某些情况下,它们之间也可能发生拮抗作用[118]。例如,乙烯转录因子EIN3 (ethylene-insensitive 3)可以与MYC2相互作用,抑制其转录活性[119, 120]。这种复杂的相互作用模式揭示了植物激素信号网络的精细调节机制,以及它们在植物免疫中的多面性角色。
此外,茉莉酸途径与其他植物激素在植物的防御机制中也存在着复杂的相互作用,其中生长素(auxin, IAA)、赤霉素(gibberellin, GA)、细胞分裂素(cytokinin, CTK)、油菜素内酯(brassinosteroid, BR)与茉莉酸途径呈现拮抗关系。IAA途径可能受到JA途径的负向调控[121],例如,过表达水稻中响应JA的MYB类转录因子JMTF1 (JA-mediating MYB transcription factor1)可以促进生长素阻遏子IAA13的表达,进而抑制IAA途径的激活[122]。相似地,在辣椒中,JA途径能够提高生长素转录负调控因子CaARF9 (auxin response factor 9)的表达水平[123],这些展现出IAA与JA途径间的相互制约。JA途径和GA途径的拮抗作用是JAZ和DELLA蛋白直接相互作用介导的,如当GA途径激活时,水稻中DELLA蛋白家族成员SLR1 (slender rice 1)能够与JA途径中的JAZ9相互作用,导致JAZ9蛋白积累进而抑制JA途径[124-126],从而在植物免疫和生长发育之间实现动态平衡。此外,JA途径可以促进GA的分解代谢,帮助植物在遭受病原菌攻击时快速调整其资源分配[127]。目前对CTK途径和JA途径相互作用的了解有限,尽管已有研究表明,CTK途径可能不直接与JA途径相互作用[128],但也有初步证据表明CTK与JA途径之间存在拮抗关系[129-131]。BR和JA途径通常呈现拮抗关系以应对病原菌威胁[132-134],如糖原合酶激酶OsGSK2 (glycogen synthase kinase 2)是水稻中BR负调节因子,它通过促进OsJAZ4蛋白的降解以及阻断OsJAZ4与OsNINJA的互作,从而促进JA信号的激活[135],而脱落酸(abscisic acid, ABA)和独脚金内酯(strigolactone, SL)通常与茉莉酸途径协同作用增强植物的抗病性[136-138]。ABA的受体PYL (pyrabactin resistance 1-like)蛋白在植物免疫中的作用不容忽视,并且PYL-MYC2复合体是ABA和JA途径协同作用发挥功能的关键模块。例如,在葡萄中沉默VvPYL4能抑制MYC2和JAR1的基因表达,进而影响葡萄对霜霉菌(Plasmopara viticola)的防御能力[139]。SL途径可以正调控JA途径的激活,从而发挥其功能[140]。在棉花(Gossypium barbadense)中,SL合成关键基因GbCCD7 (carotenoid cleavage dioxygenase 7)和GbCCD8b的过表达能够诱导GbMYC2的表达上调,从而提高了植物防御能力[141]。这些发现(表 1)不仅丰富了我们对植物激素相互作用网络的理解,也为植物抗病育种提供了新的策略和思路。
| 表 1 在植物免疫中茉莉酸途径与其他植物激素途径之间的相互作用关系 |
茉莉酸途径在植物与病原菌相互作用研究领域已取得了显著进展,不仅加深了我们对茉莉酸合成、代谢及信号转导机制的理解,还从单一的底物、酶、受体及转录因子等关键组件的发掘,逐步构建起一个全面且系统的动态模型。这一认识上的飞跃,尤其体现在茉莉酸途径在植物免疫策略中的多重角色上,从最初被视为主要应对死体营养型病原菌和害虫的笼统观念,转变为在多种病原菌防御中发挥独特作用的新视角。这一转变挑战了传统的单一防御观念,揭示了茉莉酸途径在植物与病原菌互作中的复杂性和多样性。它并非简单地遵循一套固定的模式,而是依据病原菌的类型、侵染策略及植物自身的生理状态,展现出多样化的响应策略。
展望未来,全面而深入地解析茉莉酸途径在不同植物与病原菌互作体系中的具体功能与机制,是一项充满挑战的任务。这不仅需要跨学科的协作和深入的探索,还需要深刻理解植物的复杂性和动态平衡。这样的研究有望进一步拓展我们对植物免疫系统复杂性的认识,为农业生产实践中病害防控策略的创新与优化提供理论基础和技术指导,对提高农作物的抗病性和保障农业生产的可持续性具有重要意义。
鉴于茉莉酸途径在植物抗病性中的核心作用,持续深入挖掘其关键基因,并借助基因编辑、转基因等基因工程技术,精准地改良作物遗传特性,培育出具有优良抗病性状的新品种,将是提升农业生产稳定性和可持续性的重要途径。已有研究发现,玉米3-酮酰基还原酶gl8 (glossy 8)突变体可以通过激活JA途径增强植物防御,然而这也导致作为玉米物理屏障的角质蜡的合成缺失[142]。正因为茉莉酸途径同时还广泛参与调控植物生长发育的多个关键阶段[143, 144],如种子萌发、根系发育、花果形成等,未来研究需更加注重在植物生长发育与植物免疫之间寻求精细平衡,通过对茉莉酸途径调控元件的精细调整,实现其激活时间与强度的精准控制,以避免对植物正常生长造成不利影响。
此外,考虑到植物激素信号网络之间错综复杂的相互作用,未来的研究将更加注重系统地解析茉莉酸途径与其他植物激素途径之间的协同与制衡机制,为优化农作物综合性状提供新的线索和策略。例如,西南大学裴炎团队通过启动子的理性设计,开发了能同时响应SA和JA信号的启动子,利用该启动子驱动抗菌肽表达,为多种植物提供了广谱抗病性[145]。这一创新为利用植物激素协同作用开发具有更强抗病性的作物品种开辟了新的道路。
| [1] |
张杰, 董莎萌, 王伟, 等. 植物免疫研究与抗病虫绿色防控: 进展、机遇与挑战. 中国科学: 生命科学, 2019, 49: 1479-507. |
| [2] |
Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol, 2005, 43: 205-27. DOI:10.1146/annurev.phyto.43.040204.135923 |
| [3] |
Jones JDG, Staskawicz BJ, Dangl JL. The plant immune system: from discovery to deployment. Cell, 2024, 187: 2095-116. DOI:10.1016/j.cell.2024.03.045 |
| [4] |
Dou D, Zhou JM. Phytopathogen effectors subverting host immunity: different foes, similar battleground. Cell Host Microbe, 2012, 12: 484-95. DOI:10.1016/j.chom.2012.09.003 |
| [5] |
Pieterse CMJ, Van der Does D, Zamioudis C, et al. Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol, 2012, 28: 489-521. DOI:10.1146/annurev-cellbio-092910-154055 |
| [6] |
陈金焕, 田玉如, 李艾佳, 等. 茉莉酸信号及其在木本植物中的研究进展. 中国科学: 生命科学, 2020, 50: 215-26. |
| [7] |
张乐欢, 邹昌玉, 朱天翔, 等. 茉莉酸在植物抗逆性中的研究进展. 生物工程学报, 2024, 40: 15-34. |
| [8] |
吴德伟, 汪姣姣, 谢道昕. 茉莉素与植物生物胁迫反应. 生物技术通报, 2018, 34: 14-23. |
| [9] |
吴劲松, 种康. 茉莉酸作用的分子生物学研究. 植物学通报, 2002, 19: 164-70. DOI:10.3969/j.issn.1674-3466.2002.02.005 |
| [10] |
Li M, Yu G, Cao C, et al. Metabolism, signaling, and transport of jasmonates. Plant Commun, 2021, 2: 100231. DOI:10.1016/j.xplc.2021.100231 |
| [11] |
Chauvin A, Caldelari D, Wolfender JL, et al. Four 13-lipoxygenases contribute to rapid jasmonate synthesis in wounded Arabidopsis thaliana leaves: a role for lipoxygenase 6 in responses to long-distance wound signals. New Phytol, 2013, 197: 566-75. DOI:10.1111/nph.12029 |
| [12] |
Wasternack C, Feussner I. The oxylipin pathways: biochemistry and function. Annu Rev Plant Biol, 2018, 69: 363-86. DOI:10.1146/annurev-arplant-042817-040440 |
| [13] |
Guan L, Denkert N, Eisa A, et al. JASSY, a chloroplast outer membrane protein required for jasmonate biosynthesis. Proc Natl Acad Sci U S A, 2019, 116: 10568-75. DOI:10.1073/pnas.1900482116 |
| [14] |
Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot, 2013, 111: 1021-58. DOI:10.1093/aob/mct067 |
| [15] |
Chini A, Monte I, Zamarreño AM, et al. An OPR3-independent pathway uses 4, 5-didehydrojasmonate for jasmonate synthesis. Nat Chem Biol, 2018, 14: 171-8. DOI:10.1038/nchembio.2540 |
| [16] |
Fonseca S, Chini A, Hamberg M, et al. (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol, 2009, 5: 344-50. DOI:10.1038/nchembio.161 |
| [17] |
Wasternack C, Song S. Jasmonates: biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J Exp Bot, 2017, 68: 1303-21. |
| [18] |
Li Q, Zheng J, Li S, et al. Transporter-mediated nuclear entry of jasmonoyl-isoleucine is essential for jasmonate signaling. Mol Plant, 2017, 10: 695-708. DOI:10.1016/j.molp.2017.01.010 |
| [19] |
Haroth S, Feussner K, Kelly AA, et al. The glycosyltransferase UGT76E1 significantly contributes to 12-O-glucopyranosyl- jasmonic acid formation in wounded Arabidopsis thaliana leaves. J Biol Chem, 2019, 294: 9858-72. DOI:10.1074/jbc.RA119.007600 |
| [20] |
Liu Y, Du M, Deng L, et al. MYC2 regulates the termination of jasmonate signaling via an autoregulatory negative feedback loop. Plant Cell, 2019, 31: 106-27. DOI:10.1105/tpc.18.00405 |
| [21] |
Balbi V, Devoto A. Jasmonate signalling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol, 2008, 177: 301-18. DOI:10.1111/j.1469-8137.2007.02292.x |
| [22] |
Fonseca S, Chico JM, Solano R. The jasmonate pathway: the ligand, the receptor and the core signalling module. Curr Opin Plant Biol, 2009, 12: 539-47. DOI:10.1016/j.pbi.2009.07.013 |
| [23] |
Chini A, Fonseca S, Fernández G, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature, 2007, 448: 666-71. DOI:10.1038/nature06006 |
| [24] |
Chini A, Gimenez-Ibanez S, Goossens A, et al. Redundancy and specificity in jasmonate signalling. Curr Opin Plant Biol, 2016, 33: 147-56. DOI:10.1016/j.pbi.2016.07.005 |
| [25] |
Pauwels L, Barbero GF, Geerinck J, et al. NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature, 2010, 464: 788-91. DOI:10.1038/nature08854 |
| [26] |
An C, Deng L, Zhai H, et al. Regulation of jasmonate signaling by reversible acetylation of TOPLESS in Arabidopsis. Mol Plant, 2022, 15: 1329-46. DOI:10.1016/j.molp.2022.06.014 |
| [27] |
Liu B, Seong K, Pang S, et al. Functional specificity, diversity, and redundancy of Arabidopsis JAZ family repressors in jasmonate and COI1-regulated growth, development, and defense. New Phytol, 2021, 231: 1525-45. DOI:10.1111/nph.17477 |
| [28] |
Yan J, Zhang C, Gu M, et al. The Arabidopsis coronatine insensitive1 protein is a jasmonate receptor. Plant Cell, 2009, 21: 2220-36. DOI:10.1105/tpc.109.065730 |
| [29] |
Sheard LB, Tan X, Mao H, et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature, 2010, 468: 400-5. DOI:10.1038/nature09430 |
| [30] |
Hu S, Yu K, Yan J, et al. Jasmonate perception: ligand-receptor interaction, regulation, and evolution. Mol Plant, 2023, 16: 23-42. DOI:10.1016/j.molp.2022.08.011 |
| [31] |
Wu S, Hu C, Zhu C, et al. The MYC2-PUB22-JAZ4 module plays a crucial role in jasmonate signaling in tomato. Mol Plant, 2024, 17: 598-613. DOI:10.1016/j.molp.2024.02.006 |
| [32] |
Ai D, Zhao L, You CX, et al. Apple SINA11-JAZ2 module is involved in jasmonate signaling response. J Integr Plant Biol, 2024, 66: 1270-3. DOI:10.1111/jipb.13713 |
| [33] |
Dombrecht B, Xue GP, Sprague SJ, et al. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell, 2007, 19: 2225-45. DOI:10.1105/tpc.106.048017 |
| [34] |
Fernández-Calvo P, Chini A, Fernández-Barbero G, et al. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell, 2011, 23: 701-15. DOI:10.1105/tpc.110.080788 |
| [35] |
Figueroa P, Browse J. Male sterility in Arabidopsis induced by overexpression of a MYC5-SRDX chimeric repressor. Plant J, 2015, 81: 849-60. DOI:10.1111/tpj.12776 |
| [36] |
郑嘉瑞, 杨晓燕, 叶家保, 等. MYC2转录因子在植物中的功能研究进展. 园艺学报, 2023, 50: 896-908. |
| [37] |
Goossens J, Mertens J, Goossens A. Role and functioning of bHLH transcription factors in jasmonate signalling. J Exp Bot, 2017, 68: 1333-47. |
| [38] |
Qi T, Song S, Ren Q, et al. The jasmonate-ZIM-domain proteins interact with the WD-repeat/bHLH/MYB complexes to regulate jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell, 2011, 23: 1795-814. DOI:10.1105/tpc.111.083261 |
| [39] |
Javed T, Gao SJ. WRKY transcription factors in plant defense. Trends Genet, 2023, 39: 781-801. |
| [40] |
Chen Y, Jin G, Liu M, et al. Multi-omic analyses reveal key sectors of jasmonate-mediated defense responses in rice. Plant Cell, 2024, 36: 3362-77. DOI:10.1093/plcell/koae159 |
| [41] |
Kong L, Song Q, Wei H, et al. The AP2/ERF transcription factor PtoERF15 confers drought tolerance via JA-mediated signaling in Populus. New Phytol, 2023, 240: 1848-67. DOI:10.1111/nph.19251 |
| [42] |
Lin C, Lan C, Li X, et al. A pair of nuclear factor Y transcription factors act as positive regulators in jasmonate signaling and disease resistance in Arabidopsis. J Integr Plant Biol, 2024, 66: 2042-57. DOI:10.1111/jipb.13732 |
| [43] |
Wu Q, Tong C, Chen Z, et al. NLRs derepress MED10b- and MED7-mediated repression of jasmonate-dependent transcription to activate immunity. Proc Natl Acad Sci U S A, 2023, 120: e2302226120. DOI:10.1073/pnas.2302226120 |
| [44] |
Zhai Q, Li C. The plant mediator complex and its role in jasmonate signaling. J Exp Bot, 2019, 70: 3415-24. DOI:10.1093/jxb/erz233 |
| [45] |
Zhai Q, Deng L, Li C. Mediator subunit MED25: at the nexus of jasmonate signaling. Curr Opin Plant Biol, 2020, 57: 78-86. DOI:10.1016/j.pbi.2020.06.006 |
| [46] |
Chen R, Jiang H, Li L, et al. The Arabidopsis mediator subunit MED25 differentially regulates jasmonate and abscisic acid signaling through interacting with the MYC2 and ABI5 transcription factors. Plant Cell, 2012, 24: 2898-916. DOI:10.1105/tpc.112.098277 |
| [47] |
An C, Li L, Zhai Q, et al. Mediator subunit MED25 links the jasmonate receptor to transcriptionally active chromatin. Proc Natl Acad Sci U S A, 2017, 114: e8930-9. |
| [48] |
You Y, Zhai Q, An C, et al. Leunig_homolog mediates MYC2-dependent transcriptional activation in cooperation with the coactivators HAC1 and MED25. Plant Cell, 2019, 31: 2187-205. DOI:10.1105/tpc.19.00115 |
| [49] |
Wu F, Deng L, Zhai Q, et al. Mediator subunit MED25 couples alternative splicing of JAZ genes with fine-tuning of jasmonate signaling. Plant Cell, 2019, 32: 429-48. |
| [50] |
Mengiste T. Plant immunity to necrotrophs. Annu Rev Phytopathol, 2012, 50: 267-94. DOI:10.1146/annurev-phyto-081211-172955 |
| [51] |
Wang Z, Tan X, Zhang Z, et al. Defense to Sclerotinia sclerotiorum in oilseed rape is associated with the sequential activations of salicylic acid signaling and jasmonic acid signaling. Plant Sci, 2012, 184: 75-82. DOI:10.1016/j.plantsci.2011.12.013 |
| [52] |
Ranjan A, Westrick NM, Jain S, et al. Resistance against Sclerotinia sclerotiorum in soybean involves a reprogramming of the phenylpropanoid pathway and up-regulation of antifungal activity targeting ergosterol biosynthesis. Plant Biotechnol J, 2019, 17: 1567-81. DOI:10.1111/pbi.13082 |
| [53] |
Liu D, Zhao Q, Cui X, et al. A transcriptome analysis uncovers Panax notoginseng resistance to Fusarium solani induced by methyl jasmonate. Genes Genomics, 2019, 41: 1383-96. DOI:10.1007/s13258-019-00865-z |
| [54] |
Su L, Zheng L, Wang H, et al. Panax notoginseng transcription factor WRKY15 modulates resistance to Fusarium solani by up-regulating osmotin-like protein expression and inducing JA/SA signaling pathways. BMC Plant Biol, 2023, 23: 362. DOI:10.1186/s12870-023-04373-x |
| [55] |
Ren H, Bai M, Sun J, et al. RcMYB84 and RcMYB123 mediate jasmonate-induced defense responses against Botrytis cinerea in rose (Rosa chinensis). Plant J, 2020, 103: 1839-49. DOI:10.1111/tpj.14871 |
| [56] |
Brenya E, Chen ZH, Tissue D, et al. Prior exposure of Arabidopsis seedlings to mechanical stress heightens jasmonic acid-mediated defense against necrotrophic pathogens. BMC Plant Biol, 2020, 20: 548. DOI:10.1186/s12870-020-02759-9 |
| [57] |
Sun JQ, Jiang HL, Li CY. Systemin/jasmonate-mediated systemic defense signaling in tomato. Mol Plant, 2011, 4: 607-15. DOI:10.1093/mp/ssr008 |
| [58] |
Scalschi L, Sanmartín M, Camañes G, et al. Silencing of OPR3 in tomato reveals the role of OPDA in callose deposition during the activation of defense responses against Botrytis cinerea. Plant J, 2015, 81: 304-15. DOI:10.1111/tpj.12728 |
| [59] |
Yuan HM, Liu WC, Lu YT. CATALASE2 coordinates SA-mediated repression of both auxin accumulation and JA biosynthesis in plant defenses. Cell Host Microbe, 2017, 21: 143-55. DOI:10.1016/j.chom.2017.01.007 |
| [60] |
Zhang Y, Song RF, Yuan HM, et al. Overexpressing the N-terminus of CATALASE2 enhances plant jasmonic acid biosynthesis and resistance to necrotrophic pathogen Botrytis cinerea B05.10. Mol Plant Pathol, 2021, 22: 1226-38. DOI:10.1111/mpp.13106 |
| [61] |
Yang T, Deng L, Wang Q, et al. Tomato CYP94C1 inactivates bioactive JA-Ile to attenuate jasmonate-mediated defense during fruit ripening. Mol Plant, 2024, 17: 509-12. DOI:10.1016/j.molp.2024.02.004 |
| [62] |
Zhang M, Li W, Zhang T, et al. Botrytis cinerea-induced F-box protein 1 enhances disease resistance by inhibiting JAO/JOX-mediated jasmonic acid catabolism in Arabidopsis. Mol Plant, 2024, 17: 297-311. DOI:10.1016/j.molp.2023.12.020 |
| [63] |
Wang T, Xu Y, Zhao Y, et al. Systemic screening of Fusarium oxysporum candidate effectors reveals FoSSP17 that suppresses plant immunity and contributes to virulence. Phytopathol Res, 2023, 5: 42. DOI:10.1186/s42483-023-00198-6 |
| [64] |
Du M, Zhao J, Tzeng DTW, et al. MYC2 orchestrates a hierarchical transcriptional cascade that regulates jasmonate-mediated plant immunity in tomato. Plant Cell, 2017, 29: 1883-906. DOI:10.1105/tpc.16.00953 |
| [65] |
Cui F, Li X, Wu W, et al. Ectopic expression of Botrytis susceptible1 reveals its function as a positive regulator of wound-induced cell death and plant susceptibility to Botrytis. Plant Cell, 2022, 34: 4105-16. DOI:10.1093/plcell/koac206 |
| [66] |
Chen L, Zhang L, Xiang S, et al. The transcription factor WRKY75 positively regulates jasmonate-mediated plant defense to necrotrophic fungal pathogens. J Exp Bot, 2021, 72: 1473-89. DOI:10.1093/jxb/eraa529 |
| [67] |
Zhu X, Zhao Y, Shi CM, et al. Antagonistic control of rice immunity against distinct pathogens by the two transcription modules via salicylic acid and jasmonic acid pathways. Dev Cell, 2024, 59: 1609-22. DOI:10.1016/j.devcel.2024.03.033 |
| [68] |
Thatcher LF, Manners JM, Kazan K. Fusarium oxysporum hijacks COI1-mediated jasmonate signaling to promote disease development in Arabidopsis. Plant J, 2009, 58: 927-39. DOI:10.1111/j.1365-313X.2009.03831.x |
| [69] |
Thatcher LF, Gardiner DM, Kazan K, et al. A highly conserved effector in Fusarium oxysporum is required for full virulence on Arabidopsis. Mol Plant Microbe Interact, 2012, 25: 180-90. DOI:10.1094/MPMI-08-11-0212 |
| [70] |
Shi J, Wang H, Li M, et al. Alternaria TeA toxin activates a chloroplast retrograde signaling pathway to facilitate JA-dependent pathogenicity. Plant Commun, 2024, 5: 100775. DOI:10.1016/j.xplc.2023.100775 |
| [71] |
张美祥, 杨超, 刘俊. 植物病原菌效应子. 科学通报, 2023, 68: 4895-917. |
| [72] |
Katsir L, Schilmiller AL, Staswick PE, et al. COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci U S A, 2008, 105: 7100-5. DOI:10.1073/pnas.0802332105 |
| [73] |
Geng X, Cheng J, Gangadharan A, et al. The coronatine toxin of Pseudomonas syringae is a multifunctional suppressor of Arabidopsis defense. Plant Cell, 2012, 24: 4763-74. DOI:10.1105/tpc.112.105312 |
| [74] |
Zheng XY, Spivey NW, Zeng W, et al. Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe, 2012, 11: 587-96. DOI:10.1016/j.chom.2012.04.014 |
| [75] |
Melotto M, Underwood W, Koczan J, et al. Plant stomata function in innate immunity against bacterial invasion. Cell, 2006, 126: 969-80. DOI:10.1016/j.cell.2006.06.054 |
| [76] |
Cui H, Wang Y, Xue L, et al. Pseudomonas syringae effector protein AvrB perturbs Arabidopsis hormone signaling by activating MAP kinase 4. Cell Host Microbe, 2010, 7: 164-75. DOI:10.1016/j.chom.2010.01.009 |
| [77] |
Zhou Z, Wu Y, Yang Y, et al. An Arabidopsis plasma membrane proton ATPase modulates JA signaling and is exploited by the Pseudomonas syringae effector protein AvrB for stomatal invasion. Plant Cell, 2015, 27: 2032-41. DOI:10.1105/tpc.15.00466 |
| [78] |
Jiang S, Yao J, Ma KW, et al. Bacterial effector activates jasmonate signaling by directly targeting JAZ transcriptional repressors. PLoS Pathog, 2013, 9: e1003715. DOI:10.1371/journal.ppat.1003715 |
| [79] |
Gimenez-Ibanez S, Boter M, Fernández-Barbero G, et al. The bacterial effector HopX1 targets JAZ transcriptional repressors to activate jasmonate signaling and promote infection in Arabidopsis. PLoS Biol, 2014, 12: e1001792. DOI:10.1371/journal.pbio.1001792 |
| [80] |
Yang L, Teixeira PJPL, Biswas S, et al. Pseudomonas syringae type Ⅲ effector HopBB1 promotes host transcriptional repressor degradation to regulate phytohormone responses and virulence. Cell Host Microbe, 2017, 21: 156-68. DOI:10.1016/j.chom.2017.01.003 |
| [81] |
Darino M, Chia KS, Marques J, et al. Ustilago maydis effector Jsi1 interacts with Topless corepressor, hijacking plant jasmonate/ethylene signaling. New Phytol, 2021, 229: 3393-407. DOI:10.1111/nph.17116 |
| [82] |
Wang H, Zhao D, Wei J, et al. A Phytophthora infestans RXLR effector PiAVR3b suppresses plant immunity by perturbing jasmonic acid biosynthesis. Sci Hortic, 2024, 331: 113122. DOI:10.1016/j.scienta.2024.113122 |
| [83] |
Zhao Y, Yang B, Xu H, et al. The Phytophthora effector Avh94 manipulates host jasmonic acid signaling to promote infection. J Integr Plant Biol, 2022, 64: 2199-210. DOI:10.1111/jipb.13358 |
| [84] |
Hou Y, Wang Y, Tang L, et al. SAPK10-mediated phosphorylation on WRKY72 releases its suppression on jasmonic acid biosynthesis and bacterial blight resistance. iScience, 2019, 16: 499-510. DOI:10.1016/j.isci.2019.06.009 |
| [85] |
Zhang S, Dong L, Zhang X, et al. The transcription factor GhWRKY70 from Gossypium hirsutum enhances resistance to verticillium wilt via the jasmonic acid pathway. BMC Plant Biol, 2023, 23: 141. DOI:10.1186/s12870-023-04141-x |
| [86] |
Ji M, Zhao J, Han K, et al. Turnip mosaic virus P1 suppresses JA biosynthesis by degrading cpSRP54 that delivers AOCs onto the thylakoid membrane to facilitate viral infection. PLoS Pathog, 2021, 17: e1010108. DOI:10.1371/journal.ppat.1010108 |
| [87] |
Li L, Chen J, Sun Z. Exploring the shared pathogenic strategies of independently evolved effectors across distinct plant viruses. Trends Microbiol, 2024, 32: 1021-33. DOI:10.1016/j.tim.2024.03.001 |
| [88] |
Li R, Weldegergis BT, Li J, et al. Virulence factors of geminivirus interact with MYC2 to subvert plant resistance and promote vector performance. Plant Cell, 2014, 26: 4991-5008. DOI:10.1105/tpc.114.133181 |
| [89] |
Wang J, Song W, Chai J. Structure, biochemical function, and signaling mechanism of plant NLRs. Mol Plant, 2023, 16: 75-95. DOI:10.1016/j.molp.2022.11.011 |
| [90] |
Mine A, Seyfferth C, Kracher B, et al. The defense phytohormone signaling network enables rapid, high-amplitude transcriptional reprogramming during effector-triggered immunity. Plant Cell, 2018, 30: 1199-219. DOI:10.1105/tpc.17.00970 |
| [91] |
Stegmann M, Monaghan J, Smakowska-Luzan E, et al. The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science, 2017, 355: 287-9. DOI:10.1126/science.aal2541 |
| [92] |
Guo H, Nolan TM, Song G, et al. FERONIA receptor kinase contributes to plant immunity by suppressing jasmonic acid signaling in Arabidopsis thaliana. Curr Biol, 2018, 28: 3316-24. DOI:10.1016/j.cub.2018.07.078 |
| [93] |
Xu G, Moeder W, Yoshioka K, et al. A tale of many families: calcium channels in plant immunity. Plant Cell, 2022, 34: 1551-67. DOI:10.1093/plcell/koac033 |
| [94] |
Bi G, Su M, Li N, et al. The ZAR1 resistosome is a calcium-permeable channel triggering plant immune signaling. Cell, 2021, 184: 3528-41. DOI:10.1016/j.cell.2021.05.003 |
| [95] |
Jacob P, Kim NH, Wu F, et al. Plant "helper" immune receptors are Ca2+-permeable nonselective cation channels. Science, 2021, 373: 420-5. DOI:10.1126/science.abg7917 |
| [96] |
Hu C, Wu S, Li J, et al. Herbivore-induced Ca2+ signals trigger a jasmonate burst by activating ERF16-mediated expression in tomato. New Phytol, 2022, 236: 1796-808. DOI:10.1111/nph.18455 |
| [97] |
Zhou X, Gao H, Zhang X, et al. Plant extracellular self-DNA inhibits growth and induces immunity via the jasmonate signaling pathway. Plant Physiol, 2023, 192: 2475-91. DOI:10.1093/plphys/kiad195 |
| [98] |
Hettenhausen C, Baldwin IT, Wu J. Nicotiana attenuata MPK4 suppresses a novel jasmonic acid (JA) signaling-independent defense pathway against the specialist insect Manduca sexta, but is not required for the resistance to the generalist Spodoptera littoralis. New Phytol, 2013, 199: 787-99. DOI:10.1111/nph.12312 |
| [99] |
Withers J, Yao J, Mecey C, et al. Transcription factor-dependent nuclear localization of a transcriptional repressor in jasmonate hormone signaling. Proc Natl Acad Sci U S A, 2012, 109: 20148-53. DOI:10.1073/pnas.1210054109 |
| [100] |
Zander M, Lewsey MG, Clark NM, et al. Integrated multi-omics framework of the plant response to jasmonic acid. Nat Plants, 2020, 6: 290-302. DOI:10.1038/s41477-020-0605-7 |
| [101] |
Pauwels L, Morreel K, De Witte E, et al. Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc Natl Acad Sci U S A, 2008, 105: 1380-5. DOI:10.1073/pnas.0711203105 |
| [102] |
Hillmer RA, Tsuda K, Rallapalli G, et al. The highly buffered Arabidopsis immune signaling network conceals the functions of its components. PLoS Genet, 2017, 13: e1006639. DOI:10.1371/journal.pgen.1006639 |
| [103] |
Delannoy E, Batardiere B, Pateyron S, et al. Cell specialization and coordination in Arabidopsis leaves upon pathogenic attack revealed by scRNA-seq. Plant Commun, 2023, 4: 100676. DOI:10.1016/j.xplc.2023.100676 |
| [104] |
Vogel C, Bodenhausen N, Gruissem W, et al. The Arabidopsis leaf transcriptome reveals distinct but also overlapping responses to colonization by phyllosphere commensals and pathogen infection with impact on plant health. New Phytol, 2016, 212: 192-207. DOI:10.1111/nph.14036 |
| [105] |
Spoel SH, Koornneef A, Claessens SMC, et al. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell, 2003, 15: 760-70. DOI:10.1105/tpc.009159 |
| [106] |
Meng F, Yang C, Cao J, et al. A bHLH transcription activator regulates defense signaling by nucleo-cytosolic trafficking in rice. J Integr Plant Biol, 2020, 62: 1552-73. DOI:10.1111/jipb.12922 |
| [107] |
Fang X, Chai W, Li S, et al. HSP17.4 mediates salicylic acid and jasmonic acid pathways in the regulation of resistance to Colletotrichum gloeosporioides in strawberry. Mol Plant Pathol, 2021, 22: 817-28. DOI:10.1111/mpp.13065 |
| [108] |
Ullah C, Schmidt A, Reichelt M, et al. Lack of antagonism between salicylic acid and jasmonate signalling pathways in poplar. New Phytol, 2022, 235: 701-17. DOI:10.1111/nph.18148 |
| [109] |
Geng R, Li X, Huang J, et al. The chloroplast singlet oxygen-triggered biosynthesis of salicylic acid and jasmonic acid is mediated by EX1 and GUN1 in Arabidopsis. Plant Cell Environ, 2024, 47: 2852-64. DOI:10.1111/pce.14910 |
| [110] |
Jiao L, Bian L, Luo Z, et al. Enhanced volatile emissions and anti-herbivore functions mediated by the synergism between jasmonic acid and salicylic acid pathways in tea plants. Hortic Res, 2022, 9: uhac144. DOI:10.1093/hr/uhac144 |
| [111] |
Nomoto M, Skelly MJ, Itaya T, et al. Suppression of MYC transcription activators by the immune cofactor NPR1 fine-tunes plant immune responses. Cell Rep, 2021, 37: 110125. DOI:10.1016/j.celrep.2021.110125 |
| [112] |
Zhang H, Wang F, Song W, et al. Different viral effectors suppress hormone-mediated antiviral immunity of rice coordinated by OsNPR1. Nat Commun, 2023, 14: 3011. DOI:10.1038/s41467-023-38805-x |
| [113] |
Lorenzo O, Piqueras R, Sánchez-Serrano JJ, et al. Ethylene response factor1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell, 2003, 15: 165-78. DOI:10.1105/tpc.007468 |
| [114] |
Zhu Z, An F, Feng Y, et al. Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc Natl Acad Sci U S A, 2011, 108: 12539-44. DOI:10.1073/pnas.1103959108 |
| [115] |
Song N, Wu J. Synergistic induction of phytoalexins in Nicotiana attenuata by jasmonate and ethylene signaling mediated by NaWRKY70. J Exp Bot, 2024, 75: 1063-80. DOI:10.1093/jxb/erad415 |
| [116] |
Zhou J, Mu Q, Wang X, et al. Multilayered synergistic regulation of phytoalexin biosynthesis by ethylene, jasmonate, and MAPK signaling pathways in Arabidopsis. Plant Cell, 2022, 34: 3066-87. DOI:10.1093/plcell/koac139 |
| [117] |
Hu C, Wei C, Ma Q, et al. Ethylene response factors 15 and 16 trigger jasmonate biosynthesis in tomato during herbivore resistance. Plant Physiol, 2021, 185: 1182-97. DOI:10.1093/plphys/kiaa089 |
| [118] |
Song S, Liu B, Song J, et al. A molecular framework for signaling crosstalk between jasmonate and ethylene in anthocyanin biosynthesis, trichome development, and defenses against insect herbivores in Arabidopsis. J Integr Plant Biol, 2022, 64: 1770-88. DOI:10.1111/jipb.13319 |
| [119] |
Lorenzo O, Chico JM, Sánchez-Serrano JJ, et al. Jasmonate-insensitive1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell, 2004, 16: 1938-50. DOI:10.1105/tpc.022319 |
| [120] |
Song S, Huang H, Gao H, et al. Interaction between MYC2 and ethylene insensitive3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell, 2014, 26: 263-79. DOI:10.1105/tpc.113.120394 |
| [121] |
Navarrete F, Gallei M, Kornienko AE, et al. Topless promotes plant immunity by repressing auxin signaling and is targeted by the fungal effector Naked1. Plant Commun, 2022, 3: 100269. DOI:10.1016/j.xplc.2021.100269 |
| [122] |
Uji Y, Suzuki G, Fujii Y, et al. Jasmonic acid (JA)-mediating MYB transcription factor1, JMTF1, coordinates the balance between JA and auxin signalling in the rice defence response. Physiol Plant, 2024, 176: e14257. DOI:10.1111/ppl.14257 |
| [123] |
Cai W, Tao Y, Cheng X, et al. CaIAA2-CaARF9 module mediates the trade-off between pepper growth and immunity. Plant Biotechnol J, 2024, 22: 2054-74. DOI:10.1111/pbi.14325 |
| [124] |
Um TY, Lee HY, Lee S, et al. Jasmonate ZIM-domain protein 9 interacts with slender rice 1 to mediate the antagonistic interaction between jasmonic and gibberellic acid signals in rice. Front Plant Sci, 2018, 9: 1866. DOI:10.3389/fpls.2018.01866 |
| [125] |
Yang DL, Yao J, Mei CS, et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc Natl Acad Sci U S A, 2012, 109: e1192-200. |
| [126] |
Wild M, Davière JM, Cheminant S, et al. The Arabidopsis della rga-like3 is a direct target of MYC2 and modulates jasmonate signaling responses. Plant Cell, 2012, 24: 3307-19. DOI:10.1105/tpc.112.101428 |
| [127] |
Jin G, Qi J, Zu H, et al. Jasmonate-mediated gibberellin catabolism constrains growth during herbivore attack in rice. Plant Cell, 2023, 35: 3828-44. DOI:10.1093/plcell/koad191 |
| [128] |
Naseem M, Kaltdorf M, Hussain A, et al. The impact of cytokinin on jasmonate-salicylate antagonism in Arabidopsis immunity against infection with Pst DC3000. Plant Signal Behav, 2013, 8: e26791. DOI:10.4161/psb.26791 |
| [129] |
Zhang X, Goatley M, Wang K, et al. Methyl jasmonate enhances salt stress tolerance associated with antioxidant and cytokinin alteration in perennial ryegrass. Grass Res, 2023, 3: 6. |
| [130] |
Schäfer M, Meza-Canales ID, Navarro-Quezada A, et al. Cytokinin levels and signaling respond to wounding and the perception of herbivore elicitors in Nicotiana attenuata. J Integr Plant Biol, 2015, 57: 198-212. DOI:10.1111/jipb.12227 |
| [131] |
Schäfer M, Meza-Canales ID, Brütting C, et al. Cytokinin concentrations and chase-domain-containing his kinase (NaCHK2)- and NaCHK3-mediated perception modulate herbivory-induced defense signaling and defenses in Nicotiana attenuata. New Phytol, 2015, 207: 645-58. DOI:10.1111/nph.13404 |
| [132] |
He Y, Zhang H, Sun Z, et al. Jasmonic acid-mediated defense suppresses brassinosteroid-mediated susceptibility to rice black streaked dwarf virus infection in rice. New Phytol, 2017, 214: 388-99. DOI:10.1111/nph.14376 |
| [133] |
Liao K, Peng YJ, Yuan LB, et al. Brassinosteroids antagonize jasmonate-activated plant defense responses through bri1-ems-suppressor1 (BES1). Plant Physiol, 2020, 182: 1066-82. DOI:10.1104/pp.19.01220 |
| [134] |
Ke Y, Yuan M, Liu H, et al. The versatile functions of OsALDH2B1 provide a genic basis for growth-defense trade-offs in rice. Proc Natl Acad Sci U S A, 2020, 117: 3867-73. DOI:10.1073/pnas.1918994117 |
| [135] |
He Y, Hong G, Zhang H, et al. The OsGSK2 kinase integrates brassinosteroid and jasmonic acid signaling by interacting with OsJAZ4. Plant Cell, 2020, 32: 2806-22. DOI:10.1105/tpc.19.00499 |
| [136] |
Song N, Wu J. NaWRKY70 is a key regulator of Nicotiana attenuata resistance to Alternaria alternata through regulation of phytohormones and phytoalexins biosynthesis. New Phytol, 2024, 242: 1289-306. DOI:10.1111/nph.19647 |
| [137] |
Li J, Chen L, Ding X, et al. Transcriptome analysis reveals crosstalk between the abscisic acid and jasmonic acid signaling pathways in rice-mediated defense against Nilaparvata lugens. Int J Mol Sci, 2022, 23: 6319. DOI:10.3390/ijms23116319 |
| [138] |
Qi J, Mao Y, Cui J, et al. The role of strigolactones in resistance to environmental stress in plants. Physiol Plant, 2024, 176: e14419. DOI:10.1111/ppl.14419 |
| [139] |
Liu L, Liu CY, Wang H, et al. The abscisic acid receptor gene VvPYL4 positively regulates grapevine resistance to Plasmopara viticola. Plant Cell Tiss Org, 2020, 142: 483-92. DOI:10.1007/s11240-020-01872-9 |
| [140] |
Torres-Vera R, García JM, Pozo MJ, et al. Do strigolactones contribute to plant defence?. Mol Plant Pathol, 2014, 15: 211-6. DOI:10.1111/mpp.12074 |
| [141] |
Yi F, Song A, Cheng K, et al. Strigolactones positively regulate verticillium wilt resistance in cotton via crosstalk with other hormones. Plant Physiol, 2023, 192: 945-66. DOI:10.1093/plphys/kiad053 |
| [142] |
Liu J, Li L, Xiong Z, et al. Trade-offs between the accumulation of cuticular wax and jasmonic acid-mediated herbivory resistance in maize. J Integr Plant Biol, 2024, 66: 143-59. DOI:10.1111/jipb.13586 |
| [143] |
孙雨桐, 刘德帅, 齐迅, 等. 茉莉酸调控植物生长发育和胁迫的研究进展. 生物技术通报, 2023, 39: 99-109. |
| [144] |
Ghorbel M, Brini F, Sharma A, et al. Role of jasmonic acid in plants: the molecular point of view. Plant Cell Rep, 2021, 40: 1471-94. DOI:10.1007/s00299-021-02687-4 |
| [145] |
Li X, Niu G, Fan Y, et al. Synthetic dual hormone-responsive promoters enable engineering of plants with broad-spectrum resistance. Plant Commun, 2023, 4: 100596. DOI:10.1016/j.xplc.2023.100596 |
 2025, Vol. 37
2025, Vol. 37 

 张美祥,教授,陕西师范大学生命科学学院副院长,博士生导师。围绕植物与微生物互作,主要开展以下三个方面的研究:1)植物抗病机理及抗病基因工程;2)病原菌致病成灾机理与控制;3)生防菌剂和植物免疫诱抗剂的研发与应用。研究成果以第一或通讯作者发表在Cell Host & Microbe、Molecular Plant、New Phytologist、Plant Physiology、The Plant Journal、Journal of Integrative Plant Biology、Plant Communications等国际期刊上。担任BMC Plant Biology编委,New Crops、Crop Health、《植物病理学报》和《西北植物学报》青年编委。Nature Communications、Current Biology、Trends in Plant Science、New Phytologist、PLoS Pathogens、Plant Physiology、Journal of Integrative Plant Biology等期刊审稿人。主持国家自然科学基金4项
张美祥,教授,陕西师范大学生命科学学院副院长,博士生导师。围绕植物与微生物互作,主要开展以下三个方面的研究:1)植物抗病机理及抗病基因工程;2)病原菌致病成灾机理与控制;3)生防菌剂和植物免疫诱抗剂的研发与应用。研究成果以第一或通讯作者发表在Cell Host & Microbe、Molecular Plant、New Phytologist、Plant Physiology、The Plant Journal、Journal of Integrative Plant Biology、Plant Communications等国际期刊上。担任BMC Plant Biology编委,New Crops、Crop Health、《植物病理学报》和《西北植物学报》青年编委。Nature Communications、Current Biology、Trends in Plant Science、New Phytologist、PLoS Pathogens、Plant Physiology、Journal of Integrative Plant Biology等期刊审稿人。主持国家自然科学基金4项