在C3植物中,CO2直接通过核酮糖-1, 5-二磷酸羧化酶/加氧酶(ribulose-1, 5-bisphosphate carboxylase/ oxygenase, RubisCO)的催化,在叶肉细胞(mesophyll cell, MC)中的叶绿体内被固定,形成3-磷酸甘油酸。在C4植物中,CO2的固定涉及MC和维管束鞘细胞(bundle sheath cell, BSC) 的协作。CO2 (以HCO3-形式)首先在MC中经磷酸烯醇式丙酮酸羧化酶(phosphoenolpyruvate carboxylase, PEPC)等酶的催化,形成草酰乙酸等四碳化合物,这些四碳化合物随后被运输到BSC,被NADP-苹果酸酶(NADP-dependent malate enzyme, NADP-ME)、NAD-ME或磷酸烯醇式丙酮酸羧激酶(phosphoenolpyruvate carboxykinase, PEPCK)催化脱羧后释放CO2,供那里的RubisCO再次羧化固定[1]。该过程形成了一个CO2浓缩机制(称之为C4 CO2浓缩机制,C4 CCM),使得CO2富集在BSC中的RubisCO周围。据估计,C4物种BSC内CO2浓度约为1 500 μmol·mol-1,是其MC的100~150倍[2],也远高于C3植物MC内的浓度,该浓缩机制有效减少了光呼吸造成的能量与碳损失[1, 3]。因具有CO2浓缩机制,C4植物在高温和强光等逆境条件下能保持较高的光合效率[2]。参与C4代谢的酶在MC或BSC中呈现出特异的分布[4-5],而这些酶如何实现MC和BSC特异分布是C4研究的重点(图 1)[5]。
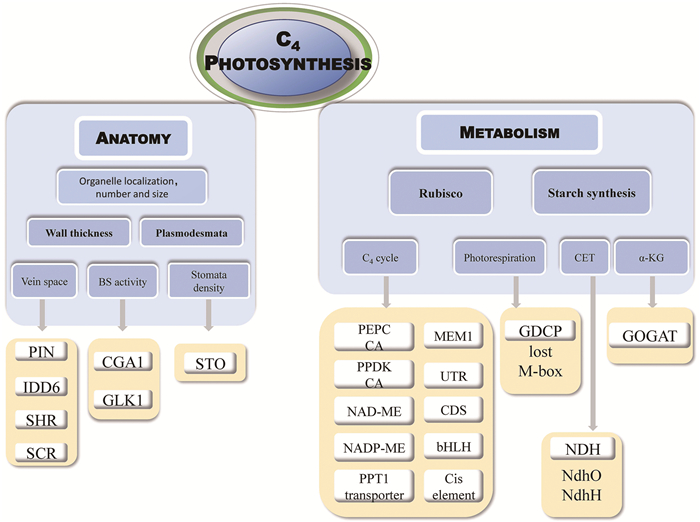
|
图示C4光合相关的结构和代谢组分,以及目前报道的相关调控机制,如C4叶片结构(anatomy)、叶脉间距(vein space)等,相关的调控因子包括PIN、IDD6、SHR和SCR等。 图 1 C4植物的解剖结构和代谢基础及调控机制 |
高等植物C4的代谢依赖于其特殊的花环结构(Kranz anatomy)[1]。C4植物BSC中含有大量叶绿体,其围绕着维管组织,在叶片横切面的解剖结构上呈现类似花环的结构,因此得名“Kranz anatomy”。C4植物BSC叶绿体的增多导致其体积增大[6],数量也增多,同时MC数量则减少;其MC与BSC紧密相连,叶片叶脉密度增加[7-9]。BSC叶绿体增加和叶脉密度增加,尤其是细小叶脉的增加,是C4结构最显著的解剖特征(图 1)[10]。
2 C4光合作用的演化 2.1 C4光合作用演化的环境因素C4植物最早出现在3千万年前的渐新世(Oligocene)[2, 11]。在单子叶植物中,C4物种主要分布在禾本科(约5 000种)和莎草科(约1 300种);而在双子叶植物中,约1 800种物种属于C4植物[2]。C4植物主要分布在温暖、干燥和热带地区,尤其是在热带草原、沙漠和亚热带地区[12-14]。历史上C4植物的两次大爆发都与大气CO2浓度的下降时间相吻合[2, 15]。根据最新地质数据,发现C4植物出现于大气CO2浓度在550 ppm以下的时期[16]。长期低浓度CO2处理使得拟南芥编码C4蛋白的同源基因表达显著上升[17-18],低CO2也可以诱导硅藻利用C4途径固定CO2[19]。因此,低CO2、高温和高光等被认为是C4光合作用演化的重要的驱动力。
2.2 C4光合作用演化的分子基础外界环境驱动并塑造了C4光合作用的演化[20],基因组水平的变化则为C4光合的演化提供了分子基础。比如,C4物种白花菜Gynandropsis gynandra,在物种形成的过程中经历了一次近期的全基因组复制事件,并在演化中保留了几乎所有来自全基因组复制产生的与叶脉发育相关的同源基因[21-22]。C4黄顶菊物种的碳酸酐酶(carbonic anhydrase, CA)、PEPC和PEPC激酶(PEPC kinase, PEPC-k)编码基因在C4物种中出现串联复制现象,显著提高了C4基因的表达水平[23]。与Gynandropsis gynandra不同,黄顶菊属的上述C4基因的复制是由转座子引起的[23]。值得一提的是,除了增加基因拷贝,转座子也为C4基因带来新的顺式调控元件,为C4基因获得新的功能提供了基础[23-24]。此外,通过比较毛颖草属(Alloteropsis)不同光合类型的物种/亚种的PEPC,发现PEPC在不同种间存在基因水平转移(lateral gene transfer, LGT)现象,而通过LGT获得的PEPC比本源的PEPC有更高的催化效率和底物亲和力[25]。因此,LGT为演化提供了一种捷径,加速了C4光合的演化[25]。综上所述,全基因组复制、串联复制、转座子和基因水平转移等都为C4光合作用的演化提供了分子基础。
2.3 C4光合作用演化的分子历程很多出现C4物种的属中含有表型介于C3和C4之间的中间型物种,通常称之为C3-C4物种。比如黄顶菊属(Flaveria)、粟米草属(Mollugo)、猪毛菜属(Salsola)、堇娘芥属 (Moricandia)、毛颖草属(Alloteropsis)等[26-27]。这些C3-C4物种CO2补偿点、水分氮素利用效率和叶脉密度都介于C3和C4物种之间,被认为是C4光合演化的中间状态。C3-C4物种为C4光合作用的演化研究提供了理想的材料。
以黄顶菊属为例,通过分析C4相关的生理表型、基因表达和蛋白质序列,结合系统发生树的分析,本团队初步绘制出了C4光合分子演化历程(图 2)。黄顶菊属中间型物种可细分为Ⅰ型C3-C4、Ⅱ型C3-C4以及类C4三种类型。Ⅰ型C3-C4物种具有所有C3-C4物种典型特征,即甘氨酸脱羧酶复合物P亚基(glycine decarboxylase complex P subunit, GDC-P)特异地在BSC中表达,将光呼吸过程中释放的CO2,以甘氨酸(glycine, Gly)形式从MC转运到BSC中释放,在BSC中实现CO2的再同化[28];因甘氨酸是2-碳有机物,该过程又被称为C2 CO2浓缩机制。在此过程中,GDC-P在BSC中催化两分子Gly生成一分子丝氨酸(serine),并产生一分子NH4+和一分子CO2,这会导致NH4+不断地从MC转移到BSC,使得MC和BSC之间的NH4+失衡[29-30]。参与C4代谢的酶,比如丙氨酸氨基转移酶(alanine aminotransferase, AlaAT)和天冬氨酸氨基转移酶(aspartate aminotransferase, AspAT),能有效地解决I型C3-C4植物Gly穿梭带来的NH4+失衡的问题,为C4代谢的发生提供了前提[30]。此外,C3-C4中间体叶脉密度比同属的C3物种增加,维管束鞘细胞中叶绿体增多,这些结构和代谢特征似乎都为C4光合的建成做了铺垫。此外,基于代谢约束模型(constraint-based)[30]、定量模型[13] 以及代谢流分析[31],绘制的C3到C3-C4再到C4代谢的演化历程,支持C3-C4代谢是C4代谢演化的过渡态的观点[32]。
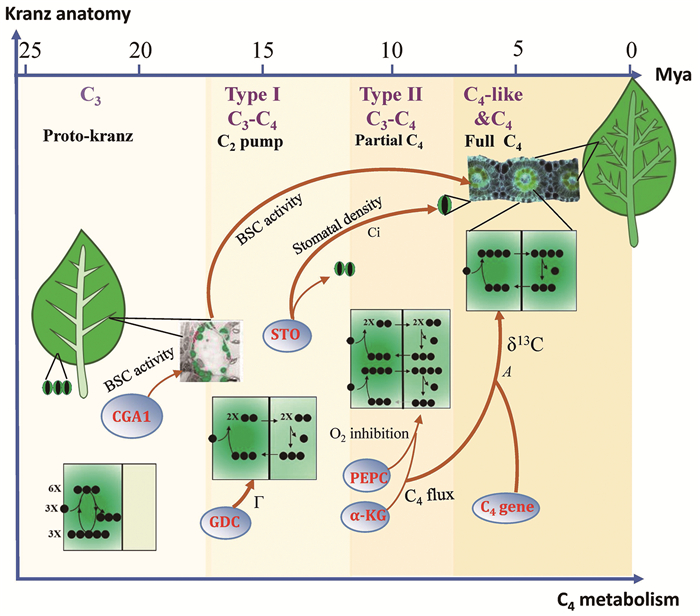
|
图示黄顶菊属中C4光合代谢和“Kranz”结构的演化历程、时间节点以及推测的内在分子机制。下方横轴代表C4代谢的演化历程,上方横轴代表物种起源的时间,纵轴代表Kranz结构的演化历程。黄顶菊属在演化的过程中依次出现C3、Ⅰ型C3-C4、Ⅱ型C3-C4、类C4 (C4-like)以及C4物种。黄顶菊属中,有些C3物种的BSC增大,其中叶绿体增多,控制该性状的基因CGA1表达水平升高,因此被认为是原始花环结构型(Proto-Kranz)。Ⅰ型C3-C4物种的主要特征是GDC-P特异性地在BSC细胞中表达,形成C2 CO2浓缩机制;此外,负责调控气孔发育的Stomagene (STO)基因表达下调,气孔密度下降。Ⅱ型C3-C4物种形成了部分C4代谢的特征,尤其是C4酶PEPC表达量提高,参与C4循环的代谢物α-酮戊二酸(α-KG)水平提高。类C4物种(C4-like)和C4物种中气孔密度显著下降,叶脉密度、C4代谢水平、光合效率以及δ13C值显著提高。值得注意的是,类C4物种和C4物种一样,具有完整的C4循环,但仍有少量CO2由RubisCO直接固定,因此类C4物种到C4物种的演化被认为是代谢的优化过程。 图 2 黄顶菊属C4光合演化分子历程的推测示意图 |
Ⅱ型C3-C4黄顶菊部分C4基因表达水平上升,尤其是CA和PEPC两个基因表达水平提高最为明显[30]。在这类物种中,约有50%的CO2 (以HCO3-形式)被PEPC固定,然而其δ13C的数值变化很小[32-33]。此外,Ⅱ型C3-C4物种叶脉密度增加,BSC相对MC的体积增大,BSC细胞中叶绿体增多,花环结构发育更趋向C4物种[34]。类C4物种已经具有较典型的花环结构和完整的C4代谢循环,但还是存在少量CO2由MC中的RubisCO直接固定[34],因此类C4到C4的演化被认为是一个代谢优化的过程[2] (图 2)。
2.4 C4光合作用演化的多途径目前报道的约50个C3-C4物种来自于20个单、双子叶属[27]。然而,并不是所有的C3-C4代谢都是C4演化的过渡态。如黄顶菊属C3-C4物种F. ramosissima中,大约50%的CO2由PEPC固定,CO2补偿点、水分利用效率、叶脉密度、气孔密度等都呈现出C3和C4的中间水平[34-35];在物种进化树上,F. ramosissima和F. trinervia的进化距离也很近[36]。因此,F. ramosissima一直被认为是Ⅱ型C3-C4物种,即不仅具有甘氨酸穿梭机制,即I型C3-C4的特征,同时还有部分C4代谢循环[37]。然而,本课题组近期通过代谢流分析发现,F. ramosissima中经PEPC催化生成的苹果酸(malate)并没有经过脱羧把CO2呈递给RubisCO,而是进入BSC线粒体生成天冬氨酸,再穿梭回MC,从而解决了C2甘氨酸穿梭代谢带来的两类细胞NH4+不平衡的问题。这种代谢途径其实位于和C4代谢平行的演化地位,而不是C4演化的过渡态[32]。
此外,毛颖草属Alloteropsis semialata的C3-C4代谢在演化上是可逆的,从C3演化而来的C3-C4代谢可以退回到C3代谢的状态[38]。因此,C3-C4植物的代谢可能是多种多样的,它们可能采用了不同的策略解决C2代谢带来的NH4+不平衡的问题,也提示C4代谢的演化存在不同途径[39]。随着更多的C3-C4代谢特征被揭示,C4演化路径也会逐渐丰富。
3 C4光合作用的遗传学 3.1 控制C4花环结构发育的机制C4植物叶片具有特殊的花环结构,其主要的两个特点是BSC中叶绿体增多和叶脉密度增加。Slewinski等[40]发现玉米scarecrow (scr)突变体中,BSC叶绿体异常分化和叶脉错乱以及叶脉密度降低。他们还发现,在SCR的互作蛋白基因short-root1 (shr1)突变的玉米叶片中Kranz结构紊乱,提出SCR/SHR信号机制可能是控制C4 “Kranz”结构发育的基础[41]。Gallagher等[42]和Wang等[43]通过比较玉米真叶(foliar leaf)和包叶(husk leaf)发育的转录组数据,进一步提出了SHR和SCR调控玉米MC和BSC发育的模型,揭示SHR和SCR是花环结构的主要调控因子[42-43]。但在C3植物拟南芥中,SCR/SHR信号是用来控制根内皮层发育的[44]。这表明,在C4演化过程中,植物可以通过招募已有的基因调控元件演化出新的功能。
Lundgren等[10]通过广泛采集和量化Alloteropsis semialata中C3、C3-C4以及C4亚种的叶片解剖特征,提出C4叶脉密度的增加主要源于细小叶脉的增加。除了SHR和SCR,Kumar等[8]推测PIN (pin-formed,生长素流出载体家族)、生长素和IDD (indeterminate domain,不定域)也参与小叶脉的发生,调控C4物种叶脉的发育。该推测在2023年的两个报道中被证实。水稻的SHR1 (short root 1,短根1)和SHR2特异分布在BSC中,直接与IDD12和IDD13互作,调控PIN的转录,从而调控叶脉的发育[45];SHR1和SHR2基因过表达可导致水稻、狗尾草和玉米的MC增加和叶脉减少,而shr1和shr2缺失突变后小叶脉数量增加[45]。在生长素处理后的SHR过表达玉米和水稻中,BSC和叶脉密度均增加,水稻叶片呈现出类C4植物的花环结构[46]。在演化过程中,C4物种不仅可以保留C3植物中已存在的SHR调控叶脉发育的机制,也通过招募新的调控途径来控制叶脉发育,比如NKD (naked-endosperm,裸胚乳)基因参与SCR调控C4物种叶脉的发育,而在C3水稻中的NKD不参与对叶脉发育的调控[47]。
在BSC叶绿体发育方面,转录因子GLK (golden2- like)的机制研究最为清楚。GLK有两个拷贝,C3物种水稻和拟南芥内源GLK1和GLK2都在MC中高表达,其功能是冗余的[43, 48]。而在玉米、狗尾草和高粱等单子叶C4物种中,GLK1主要在MC中表达,GLK2主要在BSC中表达[43, 49-50]。玉米和狗尾草glk2缺失可导致明显的生长缺陷和光合下降,而glk1单基因缺失不影响叶片叶绿体的整体发育或光系统Ⅱ功能,但GLK1过表达能部分恢复glk2的表型,表明在C4禾本科植物的演化过程中,两个基因都保留了调控叶绿体发育的功能,但GLK2在BSC叶绿体激活方面的功能更为突出[47]。双子叶植物拟南芥的GLK1和GLK2也参与光合作用基因的调控,glk1/glk2缺失的拟南芥叶片和果荚的光合作用都下降[51],表明GLK在叶绿体发育和光合基因表达调控中的功能在双子叶和单子叶物种中是保守的。Tu等[52]认为虽然GLK调控叶绿体发育和光合基因表达的功能是保守的,但是其结合的顺式调控元件在不同的物种中呈现物种特异性,而这种特异性是由基因组序列变异(cis-变异)导致的。
水稻组成型启动子UBIpro过表达玉米ZmGLK2可以增加BSC叶绿体在整个细胞中的体积占比[42],并提高其光合效率和产量[53]。此外,在表达ZmGLK1或ZmGLK2的水稻叶片中,叶绿素和色素-蛋白天线复合物的水平以及光合效率均上调,并能通过加速气孔关闭提高水稻的抗旱性[50, 54]。因此,GLK被认为是可以提高植物光合作用的“黄金”候选基因[55]。除了GLK,在水稻BSC中特异表达内源GNC (GATA, nitrate-inducible, carbon-metabolism-involved) 家族转录因子CGA1 (cytokinin GATA transcription factor 1),可使BSC叶绿体占整个细胞的体积比例增加,同时光合相关基因的表达上调,光合效率提高[56]。与GLK不同的是,GNC主要作为转录抑制因子,通过抑制光敏素互作因子(phytochrome interacting factors, PIFs) 和油菜素甾醇(brassinosteroid, BR)活性相关基因来促进叶绿体的生物发生[57]。但GNC家族的转录因子如CGA1是否在C4物种中调控叶绿体发育还有待进一步研究。
值得注意的是,目前C4花环结构形成的分子机制研究大多集中在单子叶植物中,而双子叶C4植物花环结构相关的研究还很少,双子叶和单子叶植物C4花环结构发育的分子机制是否一致还有待进一步研究。
3.2 调控C4光合代谢酶细胞特异性分布的机制 3.2.1 调控C4光合代谢酶细胞特异性分布的转录因子参与C4代谢途径的酶和转运蛋白很早就有报道[58-59],这些酶和转运蛋白如何实现在MC和BSC的特异性分布是C4光合研究的重点[5]。在单细胞测序技术成熟之前,研究者通过激光显微切割和叶片滚压等方法分离MC和BSC,再通过比较它们的转录组,获得一些在MC或BSC中特异表达的候选转录因子[60-61],进一步筛选不同C4物种保守的特异表达的转录因子[49]。通过研究发现,单/双子叶C4植物中确实存在保守的细胞特异性表达的转录因子[62]。2023年,Swift等[63]利用单细胞测序分析比较C3和C4物种MC和BSC发育轨迹的异同,认为MYB和DOF (DNA binding with one finger)可能是决定C4物种中BSC特异性的转录因子。
另一方面,研究人员也通过构建基因调控网络挖掘调控C4基因的转录因子。Tu等[64]结合原生质体ChIP-seq (chromatin immunoprecipitation,染色质免疫共沉淀技术) 和模型预测构建了玉米基因组水平的基因调控网络。本课题组基于基因共表达信息分别构建黄顶菊属C3、C3-C4中间体和C4物种中的C4基因调控网络,发现C4基因在演化过程中获得大量新的调控。此外,本课题组通过比较玉米和黄顶菊C4物种基因调控网络,还发现约40多个调控C4基因的转录因子是保守的[23]。通过体外和瞬转实验,Borba等[65]报道bHLH128和bHLH129调控玉米NADP-ME在BSC中的表达。通过分析玉米两类细胞特异的转录组数据并结合体外实验验证,Borba等[66]研究发现,DOF2和MADS1 (minichromosome maintenance factor 1/agamous/deficiens/serum response factor)可以激活玉米NADP-ME和PEPCK的表达。迄今为止,虽然对调控C4基因在MC和BSC中特异表达的转录因子已经有很多推测,但还缺乏确切的遗传学证据。
3.2.2 调控C4光合代谢酶细胞特异性分布的顺式调控元件控制C4基因在MC和BSC中特异表达的顺式调控元件已有相关报道。单子叶和双子叶植物中控制PEPC在MC中特异表达的顺式调控元件并不相同,比如MEM1 (mesophyll expression module 1)决定双子叶物种黄顶菊CA和PEPC在MC中特异高表达[67-68],而禾本科C4物种中起作用的是PEPC启动子上的四段基序[69]。G. gynandra的UTR (untranslated region,非翻译区)决定CA和PPDK (pyruvate orthophosphate dikinase,丙酮酸正磷酸双激酶)基因在MC中特异表达[70],而位于蛋白质编码区间的240 bp基序决定该物种NAD-ME (NAD-dependent malic enzyme)基因在BSC中的特异表达,类似的序列也存在于玉米和水稻NADP-ME以及拟南芥NAD-ME中[71]。此外,Borba等[65]通过体外实验表明,玉米NADP-ME启动子上被7个碱基分隔的一对顺式元件协同结合ZmbHLH128或ZmbHLH129,实现其在BSC中的特异表达[65]。这对顺式元件在黍亚科的C3和C4物种中也都存在。
在C3-C4中间型和C4物种中,GDC-P在BSC中特异表达。黄顶菊C4物种F. trinervia 和F. bidentis的GDC-P基因启动子上1 571 bp的区间负责其BSC特异性表达[72-73]。该区间分为两个关键的区域:一个是近端的R7 (约300 bp),起一般的转录增强作用;另一个是远端的R2区间(约200 bp),起到抑制基因在MC中表达的作用[72-73]。由于启动子R2区间衍生的转录本5'端内含子剪切低效,最后被降解,从而使得GDC-P在MC中表达水平很低。F. trinervia 和F. bidentis的GDC-P启动子在C3物种中也能够发挥BSC特异性表达的功能,因此常被用于在C3植物中实现目标基因的BSC特异性过表达[56, 74]。类似地,在十字花科植物中,GDC-P启动子上的M-框决定其在MC表达[75];而Moricandia属的C3-C4物种,由于GDC-P启动子上M-框丢失,使得其在MC中表达很低,从而实现了GDC-P在BSC中特异表达[76]。
组学技术的发展使得从全基因组寻找细胞特异表达的顺式调控元件成为可能。Burgess等[77]利用DNaseI-seq测序技术比较了禾本科C3和C4物种基因组染色体可及性区域的转录因子结合位点(transcription factor binding site, TFBS),报道了41个禾本科C4物种保守的BSC特异的TFBS,而这些TFBS在C3物种中不具有细胞特异性。2023年,Swift等[63]的单细胞测序结果提示,DOF家族的顺式调控元件可能与高粱BSC特异性相关。Singh等[78]通过光诱导的转录组和DNaseI-seq研究发现,双子叶C4植物G. gynandra的PPDK和PEPCK获得TGA (TGACG-binding factor motif)基序和光响应元件,如G-框和I-框,可能与它们的细胞特异表达相关。此外,Dai等[79]基于玉米MC和BSC两类细胞特异的ATAC-seq (transposase-accessible chromatin sequencing,转座酶可接近染色质测序)分析结果,发现AAAG基序(DOF家族)与BSC特异性表达相关,TGACC/T基序(WRKY家族)与MC特异性表达相关。这些预测的顺式调控元件的功能以及对应的转录因子还有待进一步的证实和研究。
3.3 获得顺式调控元件是C4基因演化的关键在C4物种演化过程中,其编码的C4基因从非光合基因变为光合基因的一部分[80],其表达和光合基因一样受光调控[81-83]。通过比较水稻和玉米黄化苗在光照转绿过程中的转录组,Xu等[81]发现,玉米C4相关基因更快地响应光强变化,可能与这些基因获得的新的顺式调控元件有关。以PPT1 (phosphoenolpyruvate transporter 1,磷酸烯醇式丙酮酸转运蛋白1)为例,从C3到C3-C4中间体到C4,PPT1转录逐渐获得光响应特征,C4物种PPT1的启动子上含有光响应顺式调控元件[84]。拟南芥编码的C4蛋白基因虽然也受光以及叶绿体到细胞核逆向信号的调控,但在其近缘C4物种G. gynandra中,这些基因受光和叶绿体信号的调控更为明显[83],其PPDK和PEPCK也获得光响应顺式调控元件,如G-框和I-框等[78]。2023年,本课题组研究发现,黄顶菊属C4物种中C4基因与光合基因的共调控,可能源于演化过程中C4基因招募了光合基因相关的顺式作用元件[32]。因此,获得新的顺式调控元件可能是C4光合基因演化获得新的调控机制的关键。
4 环式电子传递对C4光合作用的意义环式电子传递(cyclic electron transport, CET)是光合作用的一个重要组成部分,它在调节ATP和NADPH的生产比例、保护光系统以及响应环境变化中起关键作用[85-86]。C4植物,特别是NADP-ME型,通常比C3植物具有更高的CET水平,以满足C4代谢所需的额外ATP需求[87]。在C3光合作用中,叶绿体所需的ATP/NADPH比例为1.55~1.67;而在NADP-ME亚型的C4光合作用中,ATP/NADPH在MC叶绿体中的比例为1.8~1.9,在BSC叶绿体中的比例为5.0~7.5[87]。在线性电子传递中,ATP/NADPH产生比例为1.29,而在CET中仅生成ATP[88]。因此,CET还提供一种调节ATP/NADPH比例和细胞氧化还原状态的机制。故而在C4物种中,CET相关基因的表达水平高于近缘的C3物种[34, 89](图 3)。
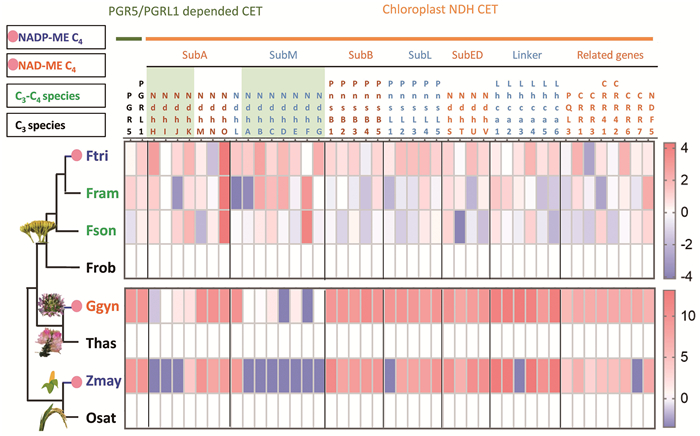
|
图中热图的颜色显示物种相对于其近缘C3物种表达水平的log2值。红色表示表达水平高于相比较的C3物种,蓝色表示低于相比较的C3物种。Flaveria trinervia (Ftri)和Zea mays (Zmay)是NADP-ME亚型C4植物,G. gynandra (Ggyn)是NAD-ME亚型C4植物。Oryza sativa (Osat)、Tarenaya hassleriana (Thas)和F. robusta (Frob)是C3物种,F. sonorensis (Fson)和F. ramosissima (Fram)是C3-C4物种。每一列表示一个蛋白,其中绿色背景表示由叶绿体基因编码(如NdhH、NdhI和NdhJ等)。不同的亚复合物,如SubA (subcomplex A)和SubB (subcomplex B)之间由黑色线条分割。 图 3 C3、C3-C4和C4物种环式电子传递相关基因表达水平比较 |
CET有两条途径,一条由PGR5 (proton gradient regulation 5)蛋白介导,另一条是由叶绿体NDH (NADH dehydrogenase)复合物介导[90]。在C3物种中,PGR5介导的CET途径发挥主要作用;而在C4物种中,尤其是NADP-ME亚型的C4物种,NDH-介导的CET途径更为重要[87, 91-92]。黄顶菊C4物种pgr5突变体的光合效率降低20%,而NDH-介导的CET相关基因ndh-o缺失后,光合效率降低80%[92]。同样,C4物种狗尾草ndh-o突变体和玉米ndh-h突变体的光合效率也都大大降低[93-94]。
在黄顶菊属中,C4物种CET相关基因的表达水平高于其同属的C3和C3-C4物种[32],在白花菜科C3和C4物种[95]以及禾本科C3和C4物种的比较研究中[81]也发现类似现象(图 3)。NDH介导的环式电子传递涉及30~40个蛋白,这些蛋白通过形成复合物行使功能,其中约10个由叶绿体基因组编码(图 3)[86]。目前环式电子传递相关基因以及蛋白质结构已有很多研究,但C4物种环式电子传递调控机制鲜有报道。
5 展望C4光合作用的研究对于理解植物适应环境、应对全球气候变化以及推动可持续农业生产意义重大。在全球为实现“双碳”目标奋斗的背景下,具有CO2浓缩机制的高效C4光合作用对实现“碳达峰”和“碳中和”的目标具有重要意义[96]。50多年来,很多研究者基于生理生态数据描绘了C4大演化(macro evolution)路径,提出了C4演化中伴随的环境因子,并明确了相关基因的多拷贝现象为C4演化提供了遗传物质基础。然而C4光合作用的分子演化,尤其是花环结构关键特征、MC和BSC蛋白特异性表达以及CET转录因子及顺式调控元件相关调控机制研究仍然处于初步阶段,一个完整的C4光合作用演化的分子历程图还需要更多的研究来描绘。未来,C4光合作用研究需要有效利用系统生物学技术和方法,系统阐明C4光合在单子叶和双子叶植物中演化的精确分子历程以及花环结构关键特征的遗传调控机制,这些都将为未来作物高光效改良以及在C3作物中实现C4光合改造提供重要指导。
致谢 感谢许大全和陈根云两位老师对本文语法瑕疵、表达不当之处进行了细致和严格的校订。| [1] |
Hatch MD. C4 photosynthesis - a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta, 1987, 895: 81-106. DOI:10.1016/S0304-4173(87)80009-5 |
| [2] |
Sage RF, Sage TL, Kocacinar F. Photorespiration and the evolution of C4 photosynthesis. Annu Rev Plant Biol, 2012, 63: 19-47. DOI:10.1146/annurev-arplant-042811-105511 |
| [3] |
Hatch MD, Slack CR. Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem J, 1966, 101: 103-11. DOI:10.1042/bj1010103 |
| [4] |
Hibberd JM, Covshoff S. The regulation of gene expression required for C4 photosynthesis. Annu Rev Plant Biol, 2010, 61: 181-207. DOI:10.1146/annurev-arplant-042809-112238 |
| [5] |
Schluter U, Weber APM. Regulation and evolution of C4 photosynthesis. Annu Rev Plant Biol, 2020, 71: 183-215. DOI:10.1146/annurev-arplant-042916-040915 |
| [6] |
McKown AD, Dengler NG. Key innovations in the evolution of Kranz anatomy and C4 vein pattern in Flaveria (Asteraceae). Am J Bot, 2007, 94: 382-99. DOI:10.3732/ajb.94.3.382 |
| [7] |
Griffiths H, Weller G, Toy LFM, et al. You're so vein: bundle sheath physiology, phylogeny and evolution in C3 and C4 plants. Plant Cell Environ, 2013, 36: 249-61. DOI:10.1111/j.1365-3040.2012.02585.x |
| [8] |
Kumar D, Kellogg EA. Getting closer: vein density in C4 leaves. New Phytol, 2019, 221: 1260-7. DOI:10.1111/nph.15491 |
| [9] |
Sedelnikova OV, Hughes TE, Langdale JA. Understanding the genetic basis of C4 Kranz anatomy with a view to engineering C3 crops. Annu Rev Genet, 2018, 52: 249-70. DOI:10.1146/annurev-genet-120417-031217 |
| [10] |
Lundgren MR, Dunning LT, Olofsson JK, et al. C4 anatomy can evolve via a single developmental change. Ecol Lett, 2019, 22: 302-12. DOI:10.1111/ele.13191 |
| [11] |
Sage RF, Christin PA, Edwards EJ. The C4 plant lineages of planet Earth. J Exp Bot, 2011, 62: 3155-69. DOI:10.1093/jxb/err048 |
| [12] |
Christin PA, Osborne CP. The recurrent assembly of C4 photosynthesis, an evolutionary tale. Photosynth Res, 2013, 117: 163-75. DOI:10.1007/s11120-013-9852-z |
| [13] |
Heckmann D, Schulze S, Denton A, et al. Predicting C4 photosynthesis evolution: modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell, 2013, 153: 1579-88. DOI:10.1016/j.cell.2013.04.058 |
| [14] |
Christin PA, Osborne CP. The evolutionary ecology of C4 plants. New Phytol, 2014, 204: 765-81. DOI:10.1111/nph.13033 |
| [15] |
Christin PA, Osborne CP, Sage RF, et al. C4 eudicots are not younger than C4 monocots. J Exp Bot, 2011, 62: 3171-81. DOI:10.1093/jxb/err041 |
| [16] |
Cenozoic CO2 Proxy Integration Project (CenCO2PIP) Consortium, Honisch B, Royer DL, et al. Toward a Cenozoic history of atmospheric CO2. Science, 2023, 382: eadi5177. DOI:10.1126/science.adi5177 |
| [17] |
Li Y, Xu J, Haq NU, et al. Was low CO2 a driving force of C4 evolution: Arabidopsis responses to long-term low CO2 stress. J Exp Bot, 2014, 65: 3657-67. DOI:10.1093/jxb/eru193 |
| [18] |
You L, Zhang JM, Li L, et al. Involvement of abscisic acid, ABI5, and PPC2 in plant acclimation to low CO2. J Exp Bot, 2020, 71: 4093-108. DOI:10.1093/jxb/eraa148 |
| [19] |
Zhang H, Zhou Y, Liu TQ, et al. Initiation of efficient C4 pathway in response to low ambient CO2 during the bloom period of a marine dinoflagellate. Environ Microbiol, 2021, 23: 3196-211. DOI:10.1111/1462-2920.15545 |
| [20] |
Sage RF, Zhu XG. Exploiting the engine of C4 photosynthesis. J Exp Bot, 2011, 62: 2989-3000. DOI:10.1093/jxb/err179 |
| [21] |
Huang CF, Liu WY, Lu MJ, et al. Whole-genome duplication facilitated the evolution of C4 photosynthesis in Gynandropsis gynandra. Mol Biol Evol, 2021, 38: 4715-31. DOI:10.1093/molbev/msab200 |
| [22] |
Hoang NV, Sogbohossou EOD, Xiong W, et al. The Gynandropsis gynandra genome provides insights into whole-genome duplications and the evolution of C4 photosynthesis in Cleomaceae. Plant Cell, 2023, 35: 1334-59. DOI:10.1093/plcell/koad018 |
| [23] |
Lyu MJ, Du H, Yao H, et al. Born with intronless ERF transcriptional factors: C4 photosynthesis inherits a legacy dating back 450 million years. BioRxiv, 2022, doi: 10.1101/2022.10.14.512192
|
| [24] |
Cao C, Xu J, Zheng G, et al. Evidence for the role of transposons in the recruitment of cis-regulatory motifs during the evolution of C4 photosynthesis. BMC Genomics, 2016, 17: 201. DOI:10.1186/s12864-016-2519-3 |
| [25] |
Phansopa C, Dunning LT, Reid JD, et al. Lateral gene transfer acts as an evolutionary shortcut to efficient C4 biochemistry. Mol Biol Evol, 2020, 37: 3094-104. DOI:10.1093/molbev/msaa143 |
| [26] |
Yu Y. Paving the way for C4 evolution: study of C3-C4 intermediate species in grasses. Plant Physiol, 2020, 182: 453-4. DOI:10.1104/pp.19.01442 |
| [27] |
Mercado MA, Studer AJ. Meeting in the middle: lessons and opportunities from studying C3-C4 intermediates. Annu Rev Plant Biol, 2022, 73: 43-65. DOI:10.1146/annurev-arplant-102720-114201 |
| [28] |
Monson RK, Edwards GE, Ku MSB. C3-C4 intermediate photosynthesis in plants. Bioscience, 1984, 34: 563-74. DOI:10.2307/1309599 |
| [29] |
Walsh CA, Brautigam A, Roberts MR, et al. Evolutionary implications of C2 photosynthesis: how complex biochemical trade-offs may limit C4 evolution. J Exp Bot, 2023, 74: 707-22. DOI:10.1093/jxb/erac465 |
| [30] |
Mallmann J, Heckmann D, Brautigam A, et al. The role of photorespiration during the evolution of C4 photosynthesis in the genus Flaveria. Elife, 2014, 3: e02478. DOI:10.7554/eLife.02478 |
| [31] |
Borghi GL, Arrivault S, Gunther M, et al. Metabolic profiles in C3, C3-C4 intermediate, C4-like, and C4 species in the genus Flaveria. J Exp Bot, 2022, 73: 1581-601. DOI:10.1093/jxb/erab540 |
| [32] |
Lyu MJ, Tang Q, Wang Y, et al. Evolution of gene regulatory network of C4 photosynthesis in the genus Flaveria reveals the evolutionary status of C3-C4 intermediate species. Plant Commun, 2023, 4: 100426. DOI:10.1016/j.xplc.2022.100426 |
| [33] |
Adachi S, Stata M, Martin DG, et al. The evolution of C4 photosynthesis in Flaveria (Asteraceae): insights from the Flaveria linearis complex. Plant Physiol, 2023, 191: 233-51. DOI:10.1093/plphys/kiac467 |
| [34] |
Lyu MA, Gowik U, Kelly S, et al. The coordination of major events in C4 photosynthesis evolution in the genus Flaveria. Sci Rep, 2021, 11: 15618. DOI:10.1038/s41598-021-93381-8 |
| [35] |
Zhao YY, Lyu MA, Miao FF, et al. The evolution of stomatal traits along the trajectory toward C4 photosynthesis. Plant Physiol, 2022, 190: 441-58. DOI:10.1093/plphys/kiac252 |
| [36] |
Lyu MJ, Gowik U, Kelly S, et al. RNA-Seq based phylogeny recapitulates previous phylogeny of the genus Flaveria (Asteraceae) with some modifications. BMC Evol Biol, 2015, 15: 116. DOI:10.1186/s12862-015-0399-9 |
| [37] |
Rumpho ME, Ku MS, Cheng SH, et al. Photosynthetic characteristics of C3-C4 intermediate Flaveria species: Ⅲ. reduction of photorespiration by a limited C4 pathway of photosynthesis in Flaveria ramosissima. Plant Physiol, 1984, 75: 993-6. DOI:10.1104/pp.75.4.993 |
| [38] |
Lundgren MR, Christin PA, Escobar EG, et al. Evolutionary implications of C3-C4 intermediates in the grass Alloteropsis semialata. Plant Cell Environ, 2016, 39: 1874-85. DOI:10.1111/pce.12665 |
| [39] |
Williams BP, Johnston IG, Covshoff S, et al. Phenotypic landscape inference reveals multiple evolutionary paths to C4 photosynthesis. Elife, 2013, 2: e00961. DOI:10.7554/eLife.00961 |
| [40] |
Slewinski TL, Anderson AA, Zhang CK, et al. Scarecrow plays a role in establishing Kranz anatomy in maize leaves. Plant Cell Physiol, 2012, 53: 2030-7. DOI:10.1093/pcp/pcs147 |
| [41] |
Slewinski TL, Anderson AA, Price S, et al. Short-root1 plays a role in the development of vascular tissue and Kranz anatomy in maize leaves. Mol Plant, 2014, 7: 1388-92. DOI:10.1093/mp/ssu036 |
| [42] |
Gallagher KL, Paquette AJ, Nakajima K, et al. Mechanisms regulating SHORT-ROOT intercellular movement. Curr Biol, 2004, 14: 1847-51. DOI:10.1016/j.cub.2004.09.081 |
| [43] |
Wang P, Khoshravesh R, Karki S, et al. Re-creation of a key step in the evolutionary switch from C3 to C4 leaf anatomy. Curr Biol, 2017, 27: 3278-87. DOI:10.1016/j.cub.2017.09.040 |
| [44] |
Wang P, Fouracre J, Kelly S, et al. Evolution of GOLDEN2- LIKE gene function in C3 and C4 plants. Planta, 2013, 237: 481-95. DOI:10.1007/s00425-012-1754-3 |
| [45] |
Liu Q, Teng S, Deng C, et al. SHORT ROOT and INDETERMINATE DOMAIN family members govern PIN-FORMED expression to regulate minor vein differentiation in rice. Plant Cell, 2023, 35: 2848-70. DOI:10.1093/plcell/koad125 |
| [46] |
Dong W, Chang T, Dai H, et al. Creating a C4-like vein pattern in rice by manipulating SHORT ROOT and auxin levels. Sci Bull (Beijing), 2023, 68: 3133-6. DOI:10.1016/j.scib.2023.10.005 |
| [47] |
Hughes TE, Sedelnikova O, Thomas M, et al. Mutations in NAKED-ENDOSPERM IDD genes reveal functional interactions with SCARECROW during leaf patterning in C4 grasses. PLoS Genet, 2023, 19: e1010715. DOI:10.1371/journal.pgen.1010715 |
| [48] |
Rossini L, Cribb L, Martin DJ, et al. The maize Golden2 gene defines a novel class of transcriptional regulators in plants. Plant Cell, 2001, 13: 1231-44. DOI:10.1105/tpc.13.5.1231 |
| [49] |
John CR, Smith-Unna RD, Woodfield H, et al. Evolutionary convergence of cell-specific gene expression in independent lineages of C4 grasses. Plant Physiol, 2014, 165: 62-75. DOI:10.1104/pp.114.238667 |
| [50] |
Li X, Wang P, Li J, et al. Maize GOLDEN2-LIKE genes enhance biomass and grain yields in rice by improving photosynthesis and reducing photoinhibition. Commun Biol, 2020, 3: 151. DOI:10.1038/s42003-020-0887-3 |
| [51] |
Zhu X, Zhang L, Kuang C, et al. Important photosynthetic contribution of silique wall to seed yield-related traits in Arabidopsis thaliana. Photosynth Res, 2018, 137: 493-501. DOI:10.1007/s11120-018-0532-x |
| [52] |
Tu X, Ren S, Shen W, et al. Limited conservation in cross-species comparison of GLK transcription factor binding suggested wide-spread cistrome divergence. Nat Commun, 2022, 13: 7632. DOI:10.1038/s41467-022-35438-4 |
| [53] |
Yeh SY, Lin HH, Chang YM, et al. Maize Golden2-like transcription factors boost rice chloroplast development, photosynthesis, and grain yield. Plant Physiol, 2022, 188: 442-59. DOI:10.1093/plphys/kiab511 |
| [54] |
Li X, Li J, Wei S, et al. Maize GOLDEN2-LIKE proteins enhance drought tolerance in rice by promoting stomatal closure. Plant Physiol, 2024, 194: 774-86. DOI:10.1093/plphys/kiad561 |
| [55] |
Hernández‐Verdeja T, Lundgren MR. GOLDEN2‐LIKE transcription factors: a golden ticket to improve crops?. Plants People Planet, 2024, 6: 79-93. DOI:10.1002/ppp3.10412 |
| [56] |
Lee DY, Hua L, Khoshravesh R, et al. Engineering chloroplast development in rice through cell-specific control of endogenous genetic circuits. Plant Biotechnol J, 2021, 19: 2291-303. DOI:10.1111/pbi.13660 |
| [57] |
Zubo YO, Blakley IC, Franco-Zorrilla JM, et al. Coordination of chloroplast development through the action of the GNC and GLK transcription factor families. Plant Physiol, 2018, 178: 130-47. DOI:10.1104/pp.18.00414 |
| [58] |
Hatch MD, Slack CR. A new enzyme for the interconversion of pyruvate and phosphopyruvate and its role in the C4 dicarboxylic acid pathway of photosynthesis. Biochem J, 1968, 106: 141-6. DOI:10.1042/bj1060141 |
| [59] |
Johnson HS, Hatch MD. The C4-dicarboxylic acid pathway of photosynthesis. Identification of intermediates and products and quantitative evidence for the route of carbon flow. Biochem J, 1969, 114: 127-34. DOI:10.1042/bj1140127 |
| [60] |
Chang YM, Liu WY, Shih AC, et al. Characterizing regulatory and functional differentiation between maize mesophyll and bundle sheath cells by transcriptomic analysis. Plant Physiol, 2012, 160: 165-77. DOI:10.1104/pp.112.203810 |
| [61] |
Wang L, Czedik-Eysenberg A, Mertz RA, et al. Comparative analyses of C4 and C3 photosynthesis in developing leaves of maize and rice. Nat Biotechnol, 2014, 32: 1158-65. DOI:10.1038/nbt.3019 |
| [62] |
Aubry S, Kelly S, Kumpers BM, et al. Deep evolutionary comparison of gene expression identifies parallel recruitment of trans-factors in two independent origins of C4 photosynthesis. PLoS Genet, 2014, 10: e1004365. DOI:10.1371/journal.pgen.1004365 |
| [63] |
Swift J, Luginbuehl LH, Schreier TB, et al. Single nuclei sequencing reveals C4 photosynthesis is based on rewiring of ancestral cell identity networks. bioRxiv, 2023, doi: 10.1101/2023.10.26.562893
|
| [64] |
Tu X, Mejia-Guerra MK, Valdes Franco JA, et al. Reconstructing the maize leaf regulatory network using ChIP-seq data of 104 transcription factors. Nat Commun, 2020, 11: 5089. DOI:10.1038/s41467-020-18832-8 |
| [65] |
Borba AR, Serra TS, Gorska A, et al. Synergistic binding of bHLH transcription factors to the promoter of the maize NADP-ME gene used in C4 photosynthesis is based on an ancient code found in the ancestral C3 state. Mol Biol Evol, 2018, 35: 1690-705. DOI:10.1093/molbev/msy060 |
| [66] |
Borba AR, Reyna-Llorens I, Dickinson PJ, et al. Compartmentation of photosynthesis gene expression in C4 maize depends on time of day. Plant Physiol, 2023, 193: 2306-20. DOI:10.1093/plphys/kiad447 |
| [67] |
Akyildiz M, Gowik U, Engelmann S, et al. Evolution and function of a cis-regulatory module for mesophyll-specific gene expression in the C4 dicot Flaveria trinervia. Plant Cell, 2007, 19: 3391-402. DOI:10.1105/tpc.107.053322 |
| [68] |
Gowik U, Schulze S, Saladie M, et al. A MEM1-like motif directs mesophyll cell-specific expression of the gene encoding the C4 carbonic anhydrase in Flaveria. J Exp Bot, 2017, 68: 311-20. DOI:10.1093/jxb/erw475 |
| [69] |
Gupta SD, Levey M, Schulze S, et al. The C4Ppc promoters of many C4 grass species share a common regulatory mechanism for gene expression in the mesophyll cell. Plant J, 2020, 101: 204-16. DOI:10.1111/tpj.14532 |
| [70] |
Williams BP, Burgess SJ, Reyna-Llorens I, et al. An untranslated cis-element regulates the accumulation of multiple C4 enzymes in Gynandropsis gynandra mesophyll cells. Plant Cell, 2016, 28: 454-65. DOI:10.1105/tpc.15.00570 |
| [71] |
Brown NJ, Newell CA, Stanley S, et al. Independent and parallel recruitment of preexisting mechanisms underlying C4 photosynthesis. Science, 2011, 331: 1436-9. DOI:10.1126/science.1201248 |
| [72] |
Engelmann S, Wiludda C, Burscheidt J, et al. The gene for the P-subunit of glycine decarboxylase from the C4 species Flaveria trinervia: analysis of transcriptional control in transgenic Flaveria bidentis (C4) and Arabidopsis (C3). Plant Physiol, 2008, 146: 1773-85. DOI:10.1104/pp.107.114462 |
| [73] |
Wiludda C, Schulze S, Gowik U, et al. Regulation of the photorespiratory GLDPA gene in C4 flaveria: an intricate interplay of transcriptional and posttranscriptional processes. Plant Cell, 2012, 24: 137-51. DOI:10.1105/tpc.111.093872 |
| [74] |
Ermakova M, Arrivault S, Giuliani R, et al. Installation of C4 photosynthetic pathway enzymes in rice using a single construct. Plant Biotechnol J, 2021, 19: 575-88. DOI:10.1111/pbi.13487 |
| [75] |
Adwy W, Laxa M, Peterhansel C. A simple mechanism for the establishment of C2-specific gene expression in Brassicaceae. Plant J, 2015, 84: 1231-8. DOI:10.1111/tpj.13084 |
| [76] |
Adwy W, Schlüter U, Papenbrock J, et al. Loss of the M-box from the glycine decarboxylase P-subunit promoter in C2 Moricandia species. Plant Gene, 2019, 18: 612-32. |
| [77] |
Burgess SJ, Reyna-Llorens I, Stevenson SR, et al. Genome-wide transcription factor binding in leaves from C3 and C4 grasses. Plant Cell, 2019, 31: 2297-314. DOI:10.1105/tpc.19.00078 |
| [78] |
Singh P, Stevenson SR, Dickinson PJ, et al. C4 gene induction during de-etiolation evolved through changes in cis to allow integration with ancestral C3 gene regulatory networks. Sci Adv, 2023, 9: eade9756. DOI:10.1126/sciadv.ade9756 |
| [79] |
Dai X, Tu X, Du B, et al. Chromatin and regulatory differentiation between bundle sheath and mesophyll cells in maize. Plant J, 2022, 109: 675-92. DOI:10.1111/tpj.15586 |
| [80] |
Aubry S, Brown NJ, Hibberd JM. The role of proteins in C3 plants prior to their recruitment into the C4 pathway. J Exp Bot, 2011, 62: 3049-59. DOI:10.1093/jxb/err012 |
| [81] |
Xu J, Brautigam A, Weber AP, et al. Systems analysis of cis-regulatory motifs in C4 photosynthesis genes using maize and rice leaf transcriptomic data during a process of de-etiolation. J Exp Bot, 2016, 67: 5105-17. DOI:10.1093/jxb/erw275 |
| [82] |
Lyu MJ, Wang Y, Jiang J, et al. What matters for C4 transporters: evolutionary changes of phosphoenolpyruvate transporter for C4 photosynthesis. Front Plant Sci, 2020, 11: 935. DOI:10.3389/fpls.2020.00935 |
| [83] |
Burgess SJ, Granero-Moya I, Grange-Guermente MJ, et al. Ancestral light and chloroplast regulation form the foundations for C4 gene expression. Nat Plants, 2016, 2: 16161. DOI:10.1038/nplants.2016.161 |
| [84] |
Reyna-Llorens I, Hibberd JM. Recruitment of pre-existing networks during the evolution of C4 photosynthesis. Philos Trans R Soc Lond B Biol Sci, 2017, 372: 20160386. DOI:10.1098/rstb.2016.0386 |
| [85] |
Pan X, Cao D, Xie F, et al. Structural basis for electron transport mechanism of complex I-like photosynthetic NAD(P)H dehydrogenase. Nat Commun, 2020, 11: 610. DOI:10.1038/s41467-020-14456-0 |
| [86] |
Shikanai T. Regulation of photosynthesis by cyclic electron transport around photosystem I. Adv Bot Res, 2020, 96: 177-204. |
| [87] |
Munekage YN, Taniguchi YY. Promotion of cyclic electron transport around photosystem I with the development of C4 photosynthesis. Plant Cell Physiol, 2016, 57: 897-903. DOI:10.1093/pcp/pcw012 |
| [88] |
Allen JF. Cyclic, pseudocyclic and noncyclic photophosphorylation: new links in the chain. Trends Plant Sci, 2003, 8: 15-9. DOI:10.1016/S1360-1385(02)00006-7 |
| [89] |
Nakamura N, Iwano M, Havaux M, et al. Promotion of cyclic electron transport around photosystem I during the evolution of NADP-malic enzyme-type C4 photosynthesis in the genus Flaveria. New Phytol, 2013, 199: 832-42. DOI:10.1111/nph.12296 |
| [90] |
Munekage Y, Hashimoto M, Miyake C, et al. Cyclic electron flow around photosystem I is essential for photosynthesis. Nature, 2004, 429: 579-82. DOI:10.1038/nature02598 |
| [91] |
Munekage YN, Taniguchi YY. A scheme for C4 evolution derived from a comparative analysis of the closely related C3, C3-C4 intermediate, C4-like, and C4 species in the genus Flaveria. Plant Mol Biol, 2022, 110: 445-54. DOI:10.1007/s11103-022-01246-z |
| [92] |
Ogawa T, Kobayashi K, Taniguchi YY, et al. Two cyclic electron flows around photosystem I differentially participate in C4 photosynthesis. Plant Physiol, 2023, 191: 2288-300. DOI:10.1093/plphys/kiad032 |
| [93] |
Ermakova M, Woodford R, Fitzpatrick D, et al. NDH complex-mediated cyclic electron flow in 1 bundle sheath cells enables C4 photosynthesis. bioRxiv, 2023, doi: 10.1101/2023.09.17.558135
|
| [94] |
Zhang Q, Tian S, Chen G, et al. Regulatory NADH dehydrogenase-like complex optimizes C4 photosynthetic carbon flow and cellular redox in maize. New Phytol, 2024, 241: 82-101. DOI:10.1111/nph.19332 |
| [95] |
Kulahoglu C, Denton AK, Sommer M, et al. Comparative transcriptome atlases reveal altered gene expression modules between two Cleomaceae C3 and C4 plant species. Plant Cell, 2014, 26: 3243-60. DOI:10.1105/tpc.114.123752 |
| [96] |
朱新广, 王佳伟, 韩斌. 植物碳汇系统与中国碳中和之路. 科学通报, 2023, 68: 12-7. |
 2024, Vol. 36
2024, Vol. 36 

 朱新广,中国科学院分子植物科学卓越创新中心研究员,博士生导师,国家“万人计划”科技创新领军人才。1996年获山东师范大学学士学位,2004年获美国伊利诺伊大学博士学位。获国际光合作用协会“卡尔文-本森”奖,主持编写《光合作用研究技术》,创立in silico Plants杂志。自2017年起担任中国科学院分子植物科学卓越创新中心光合室主任,主要从事植物高光效的改良研究,包括构建从代谢到冠层的多尺度“数字植物”模型,以及C3植物的C4光合改造研究,在Cell、PNAS、Nat Plant和Ann Rev Plant Biol等期刊发表论文160余篇
朱新广,中国科学院分子植物科学卓越创新中心研究员,博士生导师,国家“万人计划”科技创新领军人才。1996年获山东师范大学学士学位,2004年获美国伊利诺伊大学博士学位。获国际光合作用协会“卡尔文-本森”奖,主持编写《光合作用研究技术》,创立in silico Plants杂志。自2017年起担任中国科学院分子植物科学卓越创新中心光合室主任,主要从事植物高光效的改良研究,包括构建从代谢到冠层的多尺度“数字植物”模型,以及C3植物的C4光合改造研究,在Cell、PNAS、Nat Plant和Ann Rev Plant Biol等期刊发表论文160余篇