(2 中国科学院大学,北京 100101)
(2 University of Chinese Academy of Sciences, Beijing 100101, China)
1954年,美国加州大学伯克利分校的Melvin Ellis Calvin、Andrew Benson与James Alan Bassham等发表了光合作用碳循环的代谢通路、中间代谢物、还原剂以及ATP的化学计量,即光合碳还原循环,国际上英文名称为Calvin-Benson-Bassham cycle,简称为CBB循环。此综述写于该循环发现70周年之际。
光合作用是地球上最重要的固碳反应,为地球上的生命提供物质与能量[1-2]。植物与光合细菌在同化无机碳的同时,将太阳能转化为化学能并储存在所形成的有机化合物中[3]。有机物中存储的化学能是所有生命活动的主要能量来源;除了供给植物自身营养与生殖生长外,也是异养生物营养和活动所必需的。同时,光合作用通过吸收二氧化碳并释放氧气,维持大气中21%的氧含量[4-5]。因此,光合作用不仅是作物产量的基础,还维持大气中的碳、氧含量。
光合作用碳反应利用光反应及电子传递产生的同化力(ATP和NADPH),把活跃的化学能转变为稳定的化学能,储存在碳水化合物中。蓝细菌、藻类和高等植物直接利用CBB循环途径固定CO2,产生的第一个固定产物是三碳化合物3-磷酸甘油酸(PGA)。因此,这种CO2固定途径又称为C3途径或C3循环,这些使用C3途径的高等植物被称为C3植物,约占自然界中植物种类的85%[6]。另一些高等植物的碳同化在进入C3循环之前,在叶肉细胞中利用底物磷酸烯醇式丙酮酸(PEP)固定CO2 (HCO3−),产生的第一个固定产物是四碳化合物草酰乙酸(OAA),随后OAA转变为苹果酸(Malate)进入到维管束鞘细胞,释放的CO2在核酮糖-1, 5-二磷酸(RuBP)羧化酶/加氧酶(Rubisco)催化下再进入C3循环,此类植物被称为C4植物[7]。在自然界中有些高等植物在白天气孔关闭,晚上气孔打开,吸收CO2并固定为OAA,再转换为苹果酸。在白天光照条件下,苹果酸释放的CO2再经过Rubisco的作用进入C3循环,此类植物被称为景天酸代谢(crassulacean acid metabolism, CAM)植物[8-9]。虽然光合生物固定CO2的途径不同,但都需要利用C3循环合成碳水化合物。因此,C3循环是光合生物核心的固碳反应途径,每年大约有百亿吨的CO2要通过这个循环被固定[10]。本文综述光合作用C3循环及其调控,尤其是Rubisco调控。
1 卡尔文-本森-巴萨姆(CBB)循环反应20世纪早期,得益于科研人员的大量投入,光合作用研究领域快速发展。研究人员将光合作用分成光反应和暗反应[11]。术语“暗反应”(dark reaction或light-independent reaction)使用了很多年,尽管后来研究人员发现这样的命名存在一定的误导性,但是一些教材依然沿用。1947−1954年,Melvin Ellis Calvin、Andrew Benson与James Alan Bassham等在小球藻中利用放射性示踪技术和双向纸层析法技术,发表了22篇文章,详细阐述了光合作用碳反应循环的代谢通路以及各中间代谢物的化学计量[12-13]。卡尔文因此于1961年获得诺贝尔化学奖,因此很多人将光合作用碳同化途径称为卡尔文循环。
CBB循环利用光反应之后的电子传递和光合磷酸化产生的驱动同化力ATP与还原力NADPH,将空气中的CO2固定为基本的碳骨架,最终生成蔗糖、淀粉等碳水化合物,给植物自身以及其他生命提供能量与物质[14-15]。CBB循环发生在叶绿体基间质,可分为三个阶段:碳固定、碳还原和底物(RuBP)再生,包含11个酶参与的13步反应[16-17](图 1)。在碳固定阶段,无机的CO2在核酮糖-1, 5-二磷酸羧化酶/加氧酶(Rubisco)的催化作用下,羧化底物RuBP(在第二位碳原子)形成一个六碳中间产物。但这种六碳化合物极不稳定,立刻分解为两分子的三碳化合物3-磷酸甘油酸(PGA)。这一步反应将原本并不活泼的CO2分子激活,使之随后能被还原;在碳还原阶段,3-磷酸甘油酸在3-磷酸甘油酸激酶(PGK)以及3-磷酸甘油醛脱氢酶(GAPDH)的作用下还原为3-磷酸甘油醛;在底物RuBP再生阶段,一部分3-磷酸甘油醛用于合成蔗糖和淀粉,而剩下的部分经过磷酸丙糖异构酶(TPI)、1, 6-二磷酸果糖醛缩酶(FBA)、1, 6-二磷酸果糖酶(FBPase)、1, 7-二磷酸景天庚酮糖酶(SBPase)、转酮醇酶(TK)、5-磷酸核酮糖异构酶(RPE)、5-磷酸核糖异构酶(RPI)和5-磷酸核酮糖激酶(PRK)的催化最终再生成核酮糖1, 5-二磷酸。由上述三个阶段构成的CBB循环每进行一次,固定一个CO2分子,循环六次生成一分子葡萄糖。在循环中,至关重要的是在底物再生和中间产物输出之间保持平衡,因此CBB循环受到精确的调控[18-19]。
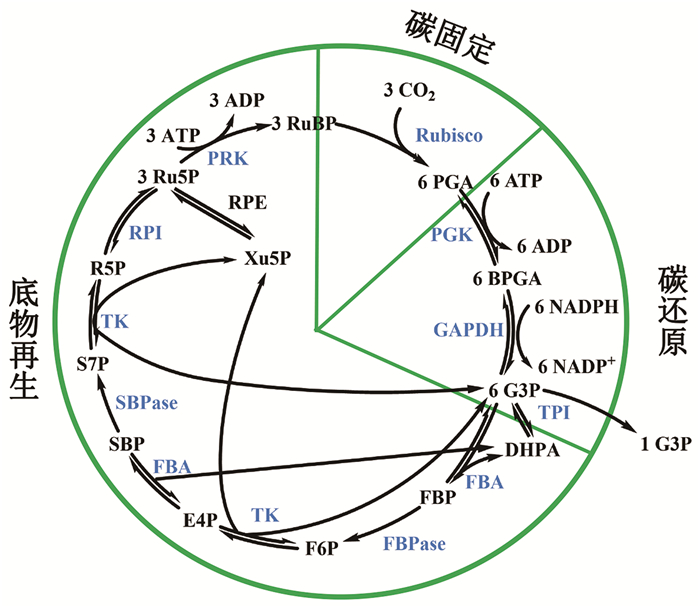
|
CBB循环分为三个阶段:碳固定、碳还原与底物再生。图中代谢中间物缩写分别为:3PGA (3-phosphoglycerate),3-磷酸甘油酸;BPGA (1, 3-diphosphoglycerate),1, 3-二磷酸甘油酸;G3P (glyceraldehyde phosphate),3-磷酸甘油醛;DHAP (dihydroxyacetone phosphate),磷酸二羟丙酮;FBP (fructose-1, 6-bisphosphate),1, 6-二磷酸果糖;F6P (fructose-6-phosphate),6-磷酸果糖;E4P (erythrose-4-phosphate),4-磷酸赤藓糖;SBP (sedoheptulose-1, 7-bisphosphate),1, 7-二磷酸景天庚酮糖;S7P (sedoheptulose-7-phosphate),7-磷酸景天庚酮糖;R5P (ribose-5-phosphate),5-磷酸核糖;Xu5P (xylulose-5-phosphate),5-磷酸木酮糖;Ru5P (ribulose 5-phosphate),5-磷酸核酮糖;RuBP (ribulose 1, 5-bisphosphate),1, 5-二磷酸核酮糖。图中酶缩写分别为:Rubisco (ribulose-1, 5-bisphosphate carboxylase/oxygenase),核酮糖-1, 5-二磷酸羧化酶/加氧酶;PGK (phosphoglycerate kinase),磷酸甘油酸激酶;GAPDH (glyderaldehyde-3-phosphate dehydrogenase),3-磷酸甘油醛脱氢酶;TPI (triose phosphate isomerase),磷酸丙糖异构酶;FBA (aldolase),1, 6-二磷酸果糖醛缩酶;FBPase (fructose-1, 6-bisphosphatase),1, 6-二磷酸果糖酶;TK (transketolase),转酮醇酶;SBPase (sedoheptulose-1, 7-bisphosphatase),1, 7-二磷酸景天庚酮糖酶;RPI (ribosephosphate isomerase),磷酸核糖异构酶;RPE (ribulose-5-phosphate-3-epimerase),磷酸核酮糖异构酶;PRK (phosphoribulokinase),磷酸核酮糖激酶。循环每进行3次,生成6分子3-磷酸甘油醛;其中有1分子3-磷酸甘油醛被运输出去用来合成其他物质,而剩下的部分经过一系列酶的催化最终再生成1, 5-二磷酸核酮糖。 图 1 光合作用CBB循环示意图 |
CBB循环没有解释循环所固定碳的去向与终产物。该循环每进行一次,固定一个CO2分子,固定的碳用于底物RuBP的再生或离开循环合成其他物质。一般认为淀粉是植物光合作用的主要产物(图 2),但由于循环代谢物再利用时产生葡萄糖,因此,很多教材中将光合作用的终产物写为葡萄糖[20]。淀粉是植物中主要的能量储存物质,为植物自身以及其他生物提供能量,也广泛应用于食品、纤维和生物燃料等工业领域。CBB循环的中间代谢物6-磷酸果糖(F6P)在磷酸葡萄糖异构酶(PGI)的作用下,产生6-磷酸葡萄糖(G6P),进而生成1-磷酸葡萄糖(G1P)以及UDP-葡萄糖(UDPG),最后在淀粉合成酶的作用下合成淀粉。光合作用组织中生成的淀粉不稳定,很快转化为单糖或者二糖被植物利用或者运输出去,因此也被称为过渡型淀粉,可用于蔗糖合成、维持植物生长发育和代谢[21-22]。在拟南芥中过表达磷酸葡萄糖异构酶的细胞质同工酶并通过信号肽定位于叶绿体内,得到的转基因植株光合作用增强,生物量增加[23]。磷酸葡萄糖变位酶(PGM)可催化1-磷酸葡萄糖和6-磷酸葡萄糖之间的相互转化,并维持其动态平衡[24-25]。在植物体中,磷酸葡萄糖变位酶分为胞质型PGM (cPGM)和质体型PGM (pPGM)。PGM参与光合作用、蔗糖代谢和淀粉合成[26]。在马铃薯中,降低cPGM的活性对其代谢影响较小,但会显著降低植物的光合作用能力[27]。

|
CO2通过CBB循环固定后产生的中间代谢物进一步用于合成其他有机物。植物主要利用CBB循环的三个代谢物:磷酸二羟丙酮、6-磷酸果糖和4-磷酸赤藓糖。代谢利用途径中间物质缩写为:PEP (phosphoenolpyruvic acid),磷酸烯醇丙酮酸;G6P (6-phosphate glucose),6-磷酸葡萄糖;G1P (1-phosphate glucose),1-磷酸葡萄糖;UDPG (UDP-glucose),尿苷二磷酸葡萄糖;Pyr (pyruvate),丙酮酸;Ac-CoA (Acetyl-CoA),乙酰辅酶A。*莽草酸途径也可能发生在质体中。 图 2 CBB循环中间代谢物的流向 |
循环中的磷酸三碳糖,如三磷酸甘油醛(G3P)、磷酸二羟丙酮(DHAP)通过三糖转运蛋白运输到细胞质中,合成类异戊二烯以及蔗糖[28]。蔗糖是从源到库长距离运输的主要形式。植物叶片和茎等进行光合作用的绿色组织称为“源”,源中合成的产物装载到韧皮部要进行长距离运输,这一过程称为“流”;到达称为“库”的不能进行光合作用的组织,包括根系、果实、种子和花等,在这里蔗糖转化为磷酸葡萄糖后产生贮藏淀粉[29]。叶绿体为具有双层被膜的细胞器,其外被膜的膜孔允许分子量小于10 kD的物质通过,但内被膜对物质的通透有很强的选择性。小分子物质可以自由通过,但ATP和葡萄糖等透过内被膜的速度很慢,而蔗糖和NADP几乎不能透过内被膜。在叶绿体的内被膜上存在一些特异的转运蛋白和离子通道,二者在控制协调间质和细胞质代谢中发挥重要作用。磷酸丙糖和三磷酸甘油酸通过叶绿体内被膜上专一的磷酸丙糖转运器(蛋白)从叶绿体向胞质转运,这种转运器被称为磷酸-磷酸丙糖/3-磷酸甘油酸转运器(Pi/triose-P/3-PGA translocator, TPT)。它能够催化磷酸、磷酸丙糖/3-PGA严格按照1:1的反向交换运输,也简称为磷酸转运器。1981年,从菠菜叶绿体被膜中分离出第一个TPT蛋白,随后其功能在植物中逐渐被揭示[30-31]。叶绿体外细胞质内高浓度的无机磷会抑制光合作用,磷浓度较低时光合过程中淀粉的合成显著增加。在马铃薯中反义抑制TPT,会导致叶片中淀粉的积累,可溶性糖含量减少[31]。在拟南芥和烟草中,反义或敲除TPT可增加碳向淀粉的分配[32],但在水稻中没有[33]。在拟南芥和烟草这些双子叶植物中,TPT活性降低时转基因植物可通过将吸收的碳转化为瞬时淀粉,来补偿TPT活性的缺乏。而在水稻叶片中主要使用蔗糖而不是淀粉作为临时储存物质,当TPT活性降低时,可能无法利用淀粉储备来循环利用Pi减轻光合抑制。
蔗糖转运蛋白(sucrose transporters, SUTs)是高等植物参与碳源分配的重要膜蛋白,在蔗糖从源到库的长距离运输过程中起关键作用[34]。植物可通过感知糖浓度的变化,重新分配碳源和调整源器官中的碳代谢,以满足库组织的需要[35]。在茄科植物中,SUT1基因的反义表达证明了其在长距离运输中的重要作用[36];在马铃薯中,反义表达StSUT1的植株光合作用减弱,导致马铃薯块茎产量降低和成龄叶片中糖分的积累[37]。在水稻中抑制OsSUT2的表达后,源端光合作用合成的蔗糖不能及时送到库组织,造成源端蔗糖大量积累,导致结实率降低和籽粒不饱满[38]。
CBB循环中另一个主要利用的中间代谢物是4-磷酸赤藓糖(E4P),其来自于CBB循环与磷酸戊糖途径,是合成芳香族氨基酸以及苯丙素的前体。莽草酸最早于1885年由Eijkman从日本植物八角的果实中分离鉴定,50年后才阐明了其结构[39]。莽草酸途径存在于细菌、真菌和植物等多种生物中,但不存在于动物中[40]。在多数细菌中,莽草酸途径主要是为蛋白质合成提供芳香族氨基酸。在植物中,莽草酸作为高等植物次生代谢的起始物,连接着糖与多酚代谢,是生物合成芳香族类氨基酸的必经途径。植物固定的碳源约有20%进入莽草酸途径,进而合成一系列次生代谢物,这些代谢物约占干重的35%。3-脱氧-D-阿拉伯庚糖酮酸-7-磷酸合成酶(DAHPS)是莽草酸合成途径的起始酶,分支酸是莽草酸途径下游连接次生代谢的枢纽物质。当分支酸合成途径受阻时,次生代谢无法顺利进行,DAHPS基因表达水平升高,会使更多的碳流向莽草酸代谢途径[41]。在水稻中,莽草酸激酶(shikimate kinase, SK)可通过控制莽草酸代谢途径来影响花器官的发育[42]。
3 植物体内CBB循环相关基因的调控植物体内碳代谢途径是交错复杂的网络,尽管光合作用CBB循环已经发现或揭示70年,但对其转运、调控以及中间代谢物的分配了解依然有限。如何提高植物同化CO2的效率,增强植物的光合作用进而提高作物产量是研究热点之一。
人们根据传统生物化学中酶的动力学特点、酶活性受多因素调控及催化反应的可逆性,推测Rubisco、1, 6-二磷酸果糖酶(FBPase)、1, 7-二磷酸景天庚酮糖酶(SBPase)和5-磷酸核酮糖激酶(PRK)是循环中关键的调控酶,这几个关键酶对光合碳同化速率有至关重要的作用。随着反义基因植物越来越多,科学家建立数学模型,计算出循环中单个酶的碳流控制系数,用其代表单个酶对CBB循环的控制程度,数值在0与1之间,数值越大对循环的调控作用越大,而且此系数随环境及发育条件的不同而改变[43]。其中,碳流控制系数较大的酶,如Rubisco、1, 6-二磷酸果糖醛缩酶(FBA)、转酮醇酶(TK)和1, 7-二磷酸景天庚酮糖酶(SBPase),被认为是改善光合作用的主要靶点。
在过量表达拟南芥1, 6-二磷酸果糖醛缩酶(FBA)的转基因烟草中,5-磷酸核酮糖及1, 5-二磷酸核酮糖的含量增加,植株的生长及其生物量均提高。这表明醛缩酶的过量表达会提高1, 5-二磷酸核酮糖的再生,从而促进CO2固定[44]。植物过量表达1, 7-二磷酸景天庚酮糖酶(SBPase)能有效增强植物的光合作用:转基因烟草在生长早期和CO2浓度升高的情况下,光合作用提高和生物量增加[45-47];转基因番茄的光合作用和耐寒应激能力增强[48];转基因水稻的耐热性提高[49];室温条件下转基因小麦的光合作用和产量增加[50]。同时过表达1, 7-二磷酸景天庚酮糖酶(SBPase)与1, 6-二磷酸果糖酶(FBPase),使高浓度CO2环境中的转基因大豆、转基因烟草的光合作用和生物量增加[51-52]。过量表达SBPase/FBPase/Ictb (inorganic carbon transporter B)的水稻和烟草、过量表达SBPase/FBA/GDCH (glycine decarboxylase-H)的拟南芥植株的光合作用和生长都增强[47, 53]。但是,并不是过表达参与CBB循环的任意酶都能够增加植物的光合作用。在水稻中过量表达转酮醇酶(TK),其活性上调80%~94%,但对水稻的光合作用、生物量和株高没有影响[54]。过量表达转酮醇酶(TK)的烟草,其酶活性上调76%~150%,但生长受到抑制并且叶片黄化[55]。这些结果表明过表达CBB循环中不同的酶对植物光合作用的影响是不同的,可能是因为某些代谢中间物产生了过量抑制效应。此外,CBB循环还受到其他因子的调控,如体内ATP与NADPH的含量、间质中其他碳水化合物的积累等[56]。
4 Rubisco对CBB循环的调控在光照充足的条件下,叶片光合作用速率受CBB循环碳反应的调控,碳反应中关键的限速步骤是Rubisco催化的碳固定反应。高等植物以及一些原核光合细菌的Rubisco是由8个大亚基和8个小亚基组成的复杂多聚体,将CO2同化到底物RuBP上是循环的第一步,也是无机碳转化为有机碳的关键枢纽[57-59]。Rubisco酶是自然界中数量最丰富的蛋白质,其含量占叶片可溶性蛋白的30%以上,可用其高含量补偿低羧化效率;大概每个分子每秒同化3个CO2分子,植物需要消耗大量能量与营养元素来合成Rubisco以实现高光合速率[60-62]。同时,Rubisco还催化底物RuBP的加氧反应,产生一分子3-磷酸甘油酸以及一分子磷酸乙醇酸,磷酸乙醇酸进入光呼吸途径,将合成的有机物又变成CO2释放到空气中,降低光合效率[63-64]。光呼吸是植物必不可少的代谢通路,适当降低光呼吸能够提高植物的光合作用[65-66]。近年来关于光呼吸通路改造方面有很多研究,钟孝芬等[67]已有详细的论述。
由于Rubisco羧化的低效以及碳固定的重要性,其一直是光合作用碳反应改造的热门靶点。早期的改造主要是对Rubisco进行诱变,以期提高酶的羧化效率以及特异性,突变位点主要在酶活性位点以及参与酶活性调控的大亚基Loop 6环序列[68]。但是,从这种突变体中没有筛选到具有高羧化且低氧化效率的Rubisco[69]。20世纪90年代,科学家在大肠杆菌中针对原核生物的Rubisco进行高通量的突变筛选,得到了表达量或稳定性提高的突变Rubisco,以及少量kcat值更高的突变Rubisco;在原核生物中表达突变的Rubisco提高了光合作用以及生物质积累[70]。目前,还没有针对高等真核生物Rubisco进行高通量筛选突变体的研究报告,原因是在大肠杆菌中组装出有功能的全酶仍比较困难。2017年,Hartl等[71]已经在大肠杆菌中组装出拟南芥Rubisco全酶,相信将来相关的研究会有所突破。2022年,科学家利用原核生物的Rubisco基因取代烟草中该基因,得到的转基因植株只能在高浓度的CO2下生长,在自然条件下无法生长[60]。Rubisco的活性位点由相邻的两个大亚基组成,小亚基的确切功能还不清楚。一般认为小亚基调控大亚基的活性,小亚基的结合不影响Rubisco的特异性[72]。将C3植物水稻Rubisco的小亚基替换为C4植物高粱的小亚基,形成的杂合Rubisco具有较高的kcat和较低的特异性(Sc/o),但Rubisco杂化导致全酶数量减少,杂合植株需要高CO2浓度才能恢复生长[73]。提高Rubisco羧化效率的另一个策略是提高体内Rubisco酶含量,进而提高植株的固碳效率。单独过表达Rubisco的大小亚基基本不影响Rubisco在植物中的含量,而如果将组装分子伴侣Raf1和Rubisco的亚基共表达,转基因植物的Rubisco含量提高超过30%,在饱和光照条件下固定CO2的量增加了15%[74]。在低光或者高CO2的环境中,电子传递速率是限制光合作用的因素,高羧化活性的Rubisco能否提高植株的生长速率还存在争议[75-77]。尽管光呼吸似乎是无用的,但其对于氮素的吸收可能是必需的,而氮素吸收利用也是植物生长的限制因素[78-80]。当其他因素不是限制条件时,Rubisco含量和羧化效率的提高以及CBB循环下游碳利用的提高,都会促进植物生长[81-82]。
Rubisco的活性严格受翻译后修饰调控。Rubisco酶进行羧化反应时,必须先活化。CO2与Mg2+结合并稳定Rubisco大亚基的保守活性位点201位赖氨酸(Lys201),使其氨甲酰化,再与底物RuBP结合进行羧化(图 3)[83-85]。Prywes等[86]详细描述了Lys201氨甲酰化的中间态、过渡态以及影响因素。质谱鉴定出Rubisco酶经历大量的翻译后修饰,这种修饰显著增加植物蛋白质组的复杂性,在植物发育调控和适应环境中发挥关键作用。在作物逆境响应和耐受性中,翻译后修饰发挥比蛋白质丰度变化更重要的作用[87]。已有研究表明,存在几种翻译后修饰作用于作物中的Rubisco大亚基(RbcL)和(或)小亚基(RbcS)[88-90]。在豆科、茄科和葫芦科的一些作物中发现一些有趣的翻译后修饰,如RbcL的第14位Lys会被高度保守的Rubisco大亚基转移酶(large subunit methyltransferase, LSMT)三甲基化。翻译后修饰可以调节植物对光信号的响应,如RbcL的乙酰化可降低黑暗中拟南芥和去黄化过程中玉米幼苗Rubisco的活性[91]。玉米Rubisco的磷酸化水平在自然日变化和去黄化过程中发生显著变化[92-93]。在拟南芥中,RbcL乙酰化水平在黑暗条件下比光照条件下高,并且乙酰化水平与Rubisco活性负相关[94]。在玉米幼苗去黄化过程中,RbcL赖氨酸乙酰化水平增加导致光照1 h后Rubisco活性降低,但随后活性水平迅速恢复[95]。拟南芥叶片和玉米叶片的蛋白提取物与大肠杆菌表达纯化的重组去乙酰化蛋白共温育可提高Rubisco活性。在弱光条件下,拟南芥缺乏叶绿体组蛋白脱乙酰酶14 (histone deacetylase 14, HDA14)可增加RbcL第474位赖氨酸乙酰化水平,提高Rubisco活性[96]。另外,在非生物胁迫下,作物中的Rubisco也存在广泛的翻译后修饰[97-101]。例如,热胁迫可使水稻RbcL去磷酸化,降低其活性[102]。在冷胁迫下,辣椒RbcL和RbcS乙酰化水平降低会限制CO2固定,导致植物的耐寒性下降[103]。敲除GNAT (general control non-repressible 5-related N-acetyltransferase,通用控制非抑制N-乙酰基转移酶)会降低番茄RbcL乙酰化水平,提高植物的耐旱性[104]。低温诱导S-亚硝基硫醇积累,促进芥菜RbcL和RbcS的硝基化,导致Rubisco活性降低[8]。豌豆(Pisum sativum L.)的镉中毒与马铃薯(Solanum tuberosum L.)和普通菜豆(Phaseolus vulgaris L.)的臭氧胁迫,均与Rubisco羰基化增加和Rubisco水平降低有关[105-107]。磷酸糖衍生物也可调节Rubisco活性[108]。在黑暗和弱光条件下,许多植物叶片中Rubisco的催化活性位点可被天然抑制剂2-羧基-d-阿拉伯糖醇-1磷酸(CA1P)紧密结合而失活[109]。
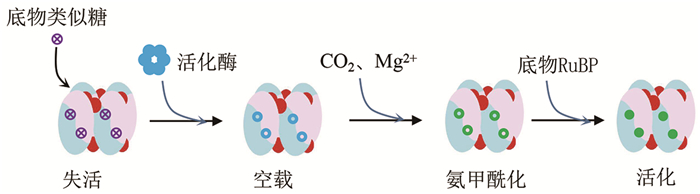
|
体内Rubisco与底物类似物(CA1P, 2-carboxy-D-arabitinol 1-phosphate)结合,处于抑制失活状态。Rubisco活化酶(Rubisco activase, RCA)结合Rubisco大亚基C末端,将其拽出,释放抑制底物后,活性中心处于空载状态。CO2与镁离子结合,Rubisco大亚基201位赖氨酸发生氨甲酰化,接受底物后发生反应。 图 3 Rubisco酶的活化 |
在自然界中,非酶因子对CBB循环也有非常强的调控作用。一个非常受关注的因子是细胞间CO2浓度(Ci),其不仅直接影响Rubisco酶的固碳效率,而且影响碳水化合物的积累[2, 110]。人们通常认为,空气中CO2浓度、气孔导度、叶肉导度以及细胞的光合能力影响Ci[111-115]。空气中CO2浓度、气孔导度增大可以使Ci增高,而叶肉细胞的光合活性升高则会导致Ci降低。
在对环境的适应中,植物演化出CO2浓缩机制(CO2 concentrating mechanism, CCM),如C4光合作用途径[7]、景天酸代谢[116],以及蓝细菌(cyanobecteria)和微藻中的无机碳吸收系统和羧酶体或淀粉核[117-118]。蓝细菌通过CO2和HCO3−高亲和摄取系统收集CO2和HCO3−,使HCO3−在细胞内累积,累积的HCO3−进入含有Rubisco的羧酶体内,被碳酸酐酶(CA)转化为CO2[119]。C4光合作用不是单一的代谢途径变化,它包括植物一系列的生化和结构上的调整,利用磷酸烯醇式丙酮酸羧化酶(PEPCase)和其他酶将CO2集中在Rubisco周围[120]。不同种类的C4植物提高Ci的具体方式可能有所不同,但基本的共同步骤是由初始羧化反应生成草酰乙酸(OAA),OAA转化为苹果酸扩散到维管束鞘细胞内进行脱羧反应,完成CO2的富集。目前,有很多研究工作尝试将蓝细菌的CCM机制引入C3植物以便提高作物光合CO2固定效率,主要通过将碳酸氢盐转运蛋白和羧酶体相关基因导入叶绿体内,并对植物细胞质内源CA的表达进行抑制,以增加HCO3−的累积。从长远来看,还需要增加更复杂的CO2泵和H+泵,以富集更多的HCO3−和维持合适的pH[121]。将C3作物的光合作用转化为C4光合作用也是人们长期的一个研究目标。虽然很多C4光合作用的酶以及碳穿梭机制在C3植物中也存在,但目前仍存在许多挑战,因为典型的C4碳浓缩循环需要跨越两种类型细胞(叶肉细胞和维管束鞘细胞),缺乏对两类细胞之间代谢物穿梭和Kranz解剖学的详细了解是主要制约因素[122]。水稻的解剖特征与C4植物最相似,如叶肉厚度相似、叶肉细胞与维管束鞘细胞的比例适中,并且维管束鞘细胞含有适量发育良好的叶绿体[123]。
CBB循环受光调节。光通过改变叶绿体内的微环境,间接影响酶的活性。光可激活磷酸甘油醛脱氢酶(GAPDH)和磷酸核酮糖激酶等参与循环的酶,在黑暗条件下这些酶会失活,这主要是由于光照对细胞内氧化还原状态的影响。硫氧还蛋白(TRXs)是一种在放氧光合生物体中普遍存在的小分子氧化还原蛋白[124]。在光照下,叶绿体中的TRXs通过铁氧还蛋白被光系统I还原,还原态的TRXs可以还原其靶蛋白上的二硫键,在大多数情况下这些靶蛋白通过还原可逆地被激活[125]。CP12是一种由大约80个氨基酸组成的内在无序蛋白(IDP),其中包括4个半胱氨酸,它们可以在TRXs的作用下形成2个二硫键[126-127]。当2个二硫键形成时,CP12可以与GAPDH和PRK组装成三元复合物,从而使两种酶失活[128-131]。通过调节其氧化还原状态,CP12可控制三元复合物的组装和拆卸,从而调节GAPDH和PRK的活性,共同调控CBB循环[132-133]。TRXs通过氧化还原调节光合机构发生和组装,这对植物的光适应、光破坏防御(photoprotection)和碳固定反应来说十分重要[134]。高温胁迫对光合机构的破坏同样会对CBB循环产生重要影响,特别是抑制循环中关键酶Rubisco的活化[135]。许多实验证明,高温降低CO2的羧化效率,主要是由于对Rubisco活性的影响[136]。Rubisco是一种双功能酶,高温胁迫抑制橡树叶片Rubisco的羧化能力[137]。Rubisco对CO2和O2的特异性,随着温度的升高而降低,进而导致植物体内羧化反应与氧化反应的比例降低。在高温胁迫下,Rubisco更倾向于发生氧化反应,产生较多的2-磷酸乙醇酸,从而进入光呼吸途径;在消耗能量的同时导致光合固碳产物的损失[138]。Rubisco活性受Rubisco活化酶(RCA)调节[76],而RCA活性受高温胁迫抑制。此外,当光合电子传递链传递的电子多于循环利用的电子时,活性氧(ROS)会在细胞内积累,如过氧化氢[139]。通过对植物Rubisco氧化还原调节的研究,人们发现Rubisco的几个半胱氨酸残基是过氧化氢等氧化剂破坏的主要靶标[140]。当Rubisco的半胱氨酸残基被氧化时,Rubisco的构象和活性改变,并且导致酶的分解代谢[141]。
6 展望随着世界人口的增长,对粮食的需求越来越大,提高农作物产量是实现国家粮食安全的重要途径。改善植物固碳效率是进一步提升作物产量的基础。与计算机模拟的理论值相比,当前作物栽培品种的固碳效率还有较大的提升空间和优化潜力,是未来作物设计改良的重点研究方向。在光照充足的条件下,CBB循环是光合作用的限速步骤。提高这个循环的效率,加强光合作用,是未来协同减少温室气体、提高作物产量的途径之一。
近年来,基因组编辑技术的快速发展,使编辑核基因组、叶绿体基因组以及线粒体基因组成为可能,为植物遗传改良提供了技术保障[142-143]。深度学习在生命科学领域如药物研发、蛋白质结构预测[144-145]方面的应用已经取得了显著成果,但是在农业上的应用还很少。AI (artificial intelligence)建立在充分的数据积累基础上,未来AI调控CBB循环的应用包括:(1) CBB循环是多酶催化的酶联反应,受复杂的环境因子与其他代谢中间物的调控,利用AI计算不同环境条件下循环的关键限制步骤,精细调控酶量或活性,提高固定CO2效率;(2)计算循环关键酶的动力学特征,预测代谢物对酶的反馈抑制作用,提高循环运转速率。
在改善CBB循环调控研究领域,未来可向如下几个方面深入。(1)搜索自然界物种,寻找关键酶的同源基因或者同工酶,计算或者建立酶活性检测体系,将高活性酶基因导入到叶绿体内,检测单个或者多个酶变量对循环的影响。(2)建立体外CBB循环体系,改变各个变量,精准鉴定调控的关键基因,利用基因组编辑或者转基因改变基因。(3)保持叶绿体间质的还原态,CBB循环的运转需要还原力,而间质的还原态有助于稳定关键酶并保持高酶活性。纳米酶(人工合成含有铁硫簇的酶)也许能够稳定保持叶绿体内的还原态。(4)引入CO2浓缩机制。因为Rubisco的低效率,适当提高Rubisco周围CO2浓度,能够增加其羧化效率,进而提高CBB循环的效率。但是,高浓度的CO2 (1%)对植物生长有抑制作用,所以,CO2浓度的调控也需精细。(5)高光效需要其他营养物质的补给,尤其是氮元素。在原核生物蓝细菌中,碳-氮平衡利用的网络比较清晰[146],但真核生物中还不明晰。总之,AI的应用和包括基因组编辑在内的合成生物学技术的发展为提高CBB循环效率启发了新的思路。
| [1] |
匡廷云, 卢从明. 加强光能高效利用机理研究的战略意义. 科学对社会的影响, 2011, 1: 23-7. |
| [2] |
张立新, 卢从明, 彭连伟, 等. 利用合成生物学原理提高光合作用效率的研究进展. 生物工程学报, 2017, 33: 486-93. |
| [3] |
Stirbet A, Lazár D, Guo Y, et al. Photosynthesis: basics, history and modelling. Ann Bot, 2020, 126: 511-37. DOI:10.1093/aob/mcz171 |
| [4] |
Hamilton TL, Bryant DA, Macalady JL. The role of biology in planetary evolution: cyanobacterial primary production in low-oxygen proterozoic oceans. Environ Microbiol, 2016, 18: 325-40. DOI:10.1111/1462-2920.13118 |
| [5] |
Tikhonov AN, Subczynski WK. Oxygenic photosynthesis: EPR study of photosynthetic electron transport and oxygen-exchange, an overview. Cell Biochem Biophys, 2019, 77: 47-59. DOI:10.1007/s12013-018-0861-6 |
| [6] |
Raines CA. Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: current and future strategies. Plant Physiol, 2011, 155: 36-42. DOI:10.1104/pp.110.168559 |
| [7] |
Furbank RT. Walking the C4 pathway: past, present, and future. J Exp Bot, 2017, 68: 4057-66. |
| [8] |
Abat JK, Deswal R. Differential modulation of S-nitrosoproteome of Brassica juncea by low temperature: change in S-nitrosylation of Rubisco is responsible for the inactivation of its carboxylase activity. Proteomics, 2009, 9: 4368-80. DOI:10.1002/pmic.200800985 |
| [9] |
章秀秀, 张锋. 景天酸代谢(CAM)植物概述. 中学生物学, 2019, 35: 3-4. |
| [10] |
Sarmiento JL. Carbon cycle atmospheric CO2 stalled. Nature, 1993, 365: 697-8. DOI:10.1038/365697a0 |
| [11] |
Blackman FF, Matthaei GLC. Experimental researches in vegetable assimilation and respiration. IV. A quantitative study of carbon-dioxide assimilation and leaf-temperature in natural illumination. Proc R Soc Lond B, 1905, 76: 402-60. DOI:10.1098/rspb.1905.0037 |
| [12] |
Bassham JA, Benson AA, Kay LD, et al. The path of carbon in photosynthesis 21. The cyclic regeneration of carbon dioxide acceptor. J Am Chem Soc, 1954, 76: 1760-70. DOI:10.1021/ja01636a012 |
| [13] |
Sharkey TD. Discovery of the canonical Calvin-Benson cycle. Photosynthesis Res, 2019, 140: 235-52. DOI:10.1007/s11120-018-0600-2 |
| [14] |
Servaites JC, Geiger DR. Regulation of ribulose 1, 5-bisphosphate carboxylase oxygenase by metabolites. J Exp Bot, 1995, 46: 1277-83. DOI:10.1093/jxb/46.special_issue.1277 |
| [15] |
Mohlmann T, Tjaden J, Henrichs G, et al. ADP-glucose drives starch synthesis in isolated maize endosperm amyloplasts: characterization of starch synthesis and transport properties across the amyloplast envelope. Biochem J, 1997, 324: 503-9. DOI:10.1042/bj3240503 |
| [16] |
Bassham JA, Benson AA, Calvin M. The path of carbon in photosynthesis. J Biol Chem, 1950, 185: 781-7. DOI:10.1016/S0021-9258(18)56368-7 |
| [17] |
Benson A, Calvin M. The dark reductions of photosynthesis. Science, 1947, 105: 648-9. DOI:10.1126/science.105.2738.648 |
| [18] |
Dijkhuizen L, Harder W. Current views on the regulation of autotrophic carbon-dioxide fixation via Calvin cycle in bacteria. Anton Leeuw J Microbio, 1984, 50: 473-87. DOI:10.1007/BF02386221 |
| [19] |
Martin W, Schnarrenberger C. The evolution of the Calvin cycle from prokaryotic to eukaryotic chromosomes: a case study of functional redundancy in ancient pathways through endosymbiosis. Curr Genet, 1997, 32: 1-18. DOI:10.1007/s002940050241 |
| [20] |
李秀豪. 光合作用产物——葡萄糖的简易证明方法. 植物生理学通讯, 1986, 1: 57. |
| [21] |
Lloyd JR, Blennow A, Burhenne K, et al. Repression of a novel isoform of disproportionating enzyme (stDPE2) in potato leads to inhibition of starch degradation in leaves but not tubers stored at low temperature. Plant Physiol, 2004, 134: 1347-54. DOI:10.1104/pp.103.038026 |
| [22] |
Zeeman SC, Smith SM, Smith AM. The diurnal metabolism of leaf starch. Biochem J, 2007, 401: 13-28. DOI:10.1042/BJ20061393 |
| [23] |
Gao F, Zhang H, Zhang W, et al. Engineering of the cytosolic form of phosphoglucose isomerase into chloroplasts improves plant photosynthesis and biomass. New Phytol, 2021, 231: 315-25. DOI:10.1111/nph.17368 |
| [24] |
Chauton MS, Winge P, Brembu T, et al. Gene regulation of carbon fixation, storage, and utilization in the diatom Phaeodactylum tricornutum acclimated to light/dark cycles. Plant Physiol, 2013, 161: 1034-48. DOI:10.1104/pp.112.206177 |
| [25] |
Uematsu K, Suzuki N, Iwamae T, et al. Expression of Arabidopsis plastidial phosphoglucomutase in tobacco stimulates photosynthetic carbon flow into starch synthesis. J Plant Physiol, 2012, 169: 1454-62. DOI:10.1016/j.jplph.2012.05.008 |
| [26] |
Paparelli E, Parlanti S, Gonzali S, et al. Nighttime sugar starvation orchestrates gibberellin biosynthesis and plant growth in Arabidopsis. Plant Cell, 2013, 25: 3760-9. DOI:10.1105/tpc.113.115519 |
| [27] |
Lytovchenko A, Sweetlove L, Pauly M, et al. The influence of cytosolic phosphoglucomutase on photosynthetic carbohydrate metabolism. Planta, 2002, 215: 1013-21. DOI:10.1007/s00425-002-0826-1 |
| [28] |
Raines CA. The Calvin cycle revisited. Photosynthesis Res, 2003, 75: 1-10. DOI:10.1023/A:1022421515027 |
| [29] |
Patrick JW, Botha FC, Birch RG. Metabolic engineering of sugars and simple sugar derivatives in plants. Plant Biotechnol J, 2013, 11: 142-56. DOI:10.1111/pbi.12002 |
| [30] |
Flugge UI, Heldt HW. The phosphate translocator of the chloroplast envelope. Isolation of the carrier protein and reconstitution of transport. Biochim Biophys Acta, 1981, 638: 296-304. DOI:10.1016/0005-2728(81)90240-1 |
| [31] |
Riesmeier JW, Flugge UI, Schulz B, et al. Antisense repression of the chloroplast triose phosphate translocator affects carbon partitioning in transgenic potato plants. Proc Natl Acad Sci U S A, 1993, 90: 6160-4. DOI:10.1073/pnas.90.13.6160 |
| [32] |
Schneider A, Häusler RE, Kolukisaoglu Ü, et al. An Arabidopsis thaliana knock-out mutant of the chloroplast triose phosphate/phosphate translocator is severely compromised only when starch synthesis, but not starch mobilisation is abolished. Plant J, 2002, 32: 685-99. DOI:10.1046/j.1365-313X.2002.01460.x |
| [33] |
Lee SK, Eom JS, Voll LM, et al. Analysis of a triose phosphate/phosphate translocator-deficient mutant reveals a limited capacity for starch synthesis in rice leaves. Mol Plant, 2014, 7: 1705-8. DOI:10.1093/mp/ssu082 |
| [34] |
Leitao L, Dizengremel P, Biolley JP. Foliar CO2 fixation in bean (Phaseolus vulgaris L.) submitted to elevated ozone: distinct changes in Rubisco and PEPc activities in relation to pigment content. Ecotoxicol Environ Saf, 2008, 69: 531-40. DOI:10.1016/j.ecoenv.2006.10.010 |
| [35] |
Vaughn MW, Harrington GN, Bush DR. Sucrose-mediated transcriptional regulation of sucrose symporter activity in the phloem. Proc Natl Acad Sci U S A, 2002, 99: 10876-80. DOI:10.1073/pnas.172198599 |
| [36] |
Riesmeier JW, Willmitzer L, Frommer WB. Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J, 1994, 13: 1-7. DOI:10.1002/j.1460-2075.1994.tb06229.x |
| [37] |
Kuhn C, Quick WP, Schulz A, et al. Companion cell-specific inhibition of the potato sucrose transporter SUT1. Plant Cell Environ, 1996, 19: 1115-23. DOI:10.1111/j.1365-3040.1996.tb00426.x |
| [38] |
Sun AJ, Dai Y, Zhang XS, et al. A transgenic study on affecting potato tuber yield by expressing the rice sucrose transporter genes OsSUT5Z and OsSUT2M. J Integr Plant Biol, 2011, 53: 586-95. DOI:10.1111/j.1744-7909.2011.01063.x |
| [39] |
Enrich LB, Scheuermann ML, Mohadjer A, et al. Liquidambar styraciflua: a renewable source of shikimic acid. Tetrahedron Lett, 2008, 49: 2503-5. DOI:10.1016/j.tetlet.2008.02.140 |
| [40] |
Herrmann KM. The shikimate pathway early steps in the biosynthesis of aromatic compounds. Plant Cell, 1995, 7: 907-19. DOI:10.2307/3870046 |
| [41] |
Janzik I, Preiskowski S, Kneifel H. Ozone has dramatic effects on the regulation of the prechorismate pathway in tobacco (Nicotiana tabacum L. cv. Bel W3). Planta, 2005, 223: 20-7. DOI:10.1007/s00425-005-0060-8 |
| [42] |
Kasai K, Kanno T, Akita M, et al. Identification of three shikimate kinase genes in rice: characterization of their differential expression during panicle development and of the enzymatic activities of the encoded proteins. Planta, 2005, 222: 438-47. DOI:10.1007/s00425-005-1559-8 |
| [43] |
Zhu XG, Long SP, Ort DR. Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol, 2010, 61: 235-61. DOI:10.1146/annurev-arplant-042809-112206 |
| [44] |
Uematsu K, Suzuki N, I T, et al. Increased fructose 1, 6-bisphosphate aldolase in plastids enhances growth and photosynthesis of tobacco plants. J Exp Bot, 2012, 63: 3001-9. DOI:10.1093/jxb/ers004 |
| [45] |
Lefebvre S, Lawson T, Zakhleniuk OV, et al. Increased sedoheptulose-1, 7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol, 2005, 138: 451-60. DOI:10.1104/pp.104.055046 |
| [46] |
Lopez-Calcagno PE, Brown KL, Simkin AJ, et al. Stimulating photosynthetic processes increases productivity and water-use efficiency in the field. Nat Plants, 2020, 6: 1054-63. DOI:10.1038/s41477-020-0740-1 |
| [47] |
Simkin AJ, Lopez-Calcagno PE, Davey PA, et al. Simultaneous stimulation of sedoheptulose 1, 7-bisphosphatase, fructose 1, 6-bisphophate aldolase and the photorespiratory glycine decarboxylase-H protein increases CO2 assimilation, vegetative biomass and seed yield in Arabidopsis. Plant Biotechnol J, 2017, 15: 805-16. DOI:10.1111/pbi.12676 |
| [48] |
Ding F, Wang ML, Zhang SX, et al. Changes in SBPase activity influence photosynthetic capacity, growth, and tolerance to chilling stress in transgenic tomato plants. Sci Rep, 2016, 6: 32741. DOI:10.1038/srep32741 |
| [49] |
Feng L, Wang K, Li Y, et al. Overexpression of SBPase enhances photosynthesis against high temperature stress in transgenic rice plants. Plant Cell Rep, 2007, 26: 1635-46. DOI:10.1007/s00299-006-0299-y |
| [50] |
Driever SM, Simkin AJ, Alotaibi S, et al. Increased SBPase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions. Philo T Roy Soc B, 2017, 372: 20160384. DOI:10.1098/rstb.2016.0384 |
| [51] |
Kohler IH, Ruiz-Vera UM, VanLoocke A, et al. Expression of cyanobacterial FBP/SBPase in soybean prevents yield depression under future climate conditions. J Exp Bot, 2017, 68: 715-26. |
| [52] |
Miyagawa Y, Tamoi M, Shigeoka S. Overexpression of a cyanobacterial fructose-1, 6-/sedoheptulose-1, 7-bisphosphatase in tobacco enhances photosynthesis and growth. Nat Biotechnol, 2001, 19: 965-9. DOI:10.1038/nbt1001-965 |
| [53] |
Gong HY, Li Y, Fang G, et al. Transgenic rice expressing Ictb and FBP sbpase derived from cyanobacteria exhibits enhanced photosynthesis and mesophyll conductance to CO2. Plos One, 2015, 10: e0140928. DOI:10.1371/journal.pone.0140928 |
| [54] |
Suzuki Y, Kondo E, Makino A. Effects of co-overexpression of the genes of Rubisco and transketolase on photosynthesis in rice. Photosynthesis Res, 2017, 131: 281-9. DOI:10.1007/s11120-016-0320-4 |
| [55] |
Khozaei M, Fisk S, Lawson T, et al. Overexpression of plastid transketolase in tobacco results in a thiamine auxotrophic phenotype. Plant Cell, 2015, 27: 432-47. DOI:10.1105/tpc.114.131011 |
| [56] |
翁晓燕, 陆庆. 水稻Rubisco活化酶在调节Rubisco活性和光合日变化中的作用. 中国水稻科学, 2001, 15: 35-40. |
| [57] |
Bar-On YM, Milo R. The global mass and average rate of Rubisco. Proc Natl Acad Sci U S A, 2019, 116: 4738-43. DOI:10.1073/pnas.1816654116 |
| [58] |
Berg IA. Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl Environ Microbiol, 2011, 77: 1925-36. DOI:10.1128/AEM.02473-10 |
| [59] |
Field CB, Behrenfeld MJ, Randerson JT, et al. Primary production of the biosphere: integrating terrestrial and oceanic components. Science, 1998, 281: 237-40. DOI:10.1126/science.281.5374.237 |
| [60] |
Liu LN. Advances in the bacterial organelles for CO2 fixation. Trends Microbiol, 2022, 30: 567-80. DOI:10.1016/j.tim.2021.10.004 |
| [61] |
Kumar S. Structure and functional annotation of hypothetical proteins having putative Rubisco activase function from Vitis vinifera. Bioinformation, 2015, 11: 11-6. DOI:10.6026/97320630011011 |
| [62] |
Sui N, Huang F, Liu LN. Photosynthesis in phytoplankton: insights from the newly discovered biological inorganic carbon pumps. Mol Plant, 2020, 13: 949-51. DOI:10.1016/j.molp.2020.05.003 |
| [63] |
South PF, Cavanagh AP, Liu HW, et al. Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science, 2019, 363: eaat9077. DOI:10.1126/science.aat9077 |
| [64] |
Whitney SM, Birch R, Kelso C, et al. Improving recombinant Rubisco biogenesis, plant photosynthesis and growth by coexpressing its ancillary RAF1 chaperone. Proc Natl Acad Sci U S A, 2015, 112: 3564-9. DOI:10.1073/pnas.1420536112 |
| [65] |
Noelke G, Houdelet M, Kreuzaler F, et al. The expression of a recombinant glycolate dehydrogenase polyprotein in potato (Solanum tuberosum) plastids strongly enhances photosynthesis and tuber yield. Plant Biotechnol J, 2014, 12: 734-42. DOI:10.1111/pbi.12178 |
| [66] |
Maier A, Fahnenstich H, von Caemmerer S, et al. Transgenic introduction of a glycolate oxidative cycle into Athaliana chloroplasts leads to growth improvement. Front Plant Sci, 2012, 3: 38. |
| [67] |
钟孝芬, 李波娣, 李敏姬, 等. 光呼吸研究进展. 热带亚热带植物学报, 2022, 30: 782-90. |
| [68] |
Andersson I, Backlund A. Structure and function of Rubisco. Plant Physiol Biochem, 2008, 46: 275-91. DOI:10.1016/j.plaphy.2008.01.001 |
| [69] |
Gutteridge S, Lorimer G, Pierce J. Details of the reactions catalyzed by mutant forms of Rubisco. Plant Physiol Biochem, 1988, 26: 675-82. |
| [70] |
Bainbridge G, Madgwick P, Parmar S, et al. Engineering Rubisco to change its catalytic properties. J Exp Bot, 1995, 46: 1269-76. DOI:10.1093/jxb/46.special_issue.1269 |
| [71] |
Hartl A, Wilson RH, Bracher A, et al. Plant Rubisco assembly in E. coli with five chloroplast chaperones including BSD2. Science, 2017, 358: 1272-8. DOI:10.1126/science.aap9221 |
| [72] |
Schulz L, Guo Z, Zarzycki J, et al. Evolution of increased complexity and specificity at the dawn of form I Rubiscos. Science, 2022, 378: 155-60. DOI:10.1126/science.abq1416 |
| [73] |
Matsumura H, Shiomi K, Yamamoto A, et al. Hybrid Rubisco with complete replacement of rice Rubisco small subunits by Sorghum counterparts confers C4 plant-like high catalytic activity. Mol Plant, 2020, 13: 1570-81. DOI:10.1016/j.molp.2020.08.012 |
| [74] |
Salesse-Smith CE, Sharwood RE, Busch FA, et al. Overexpression of Rubisco subunits with RAF1 increases Rubisco content in maize. Nat Plants, 2018, 4: 802-10. DOI:10.1038/s41477-018-0252-4 |
| [75] |
Lin MT, Occhialini A, Andralojc PJ, et al. β-Carboxysomal proteins assemble into highly organized structures in Nicotiana chloroplasts. Plant J, 2014, 79: 1-12. DOI:10.1111/tpj.12536 |
| [76] |
Zhu XG, Portis AR, Long SP. Would transformation of C3 crop plants with foreign Rubisco increase productivity? A computational analysis extrapolating from kinetic properties to canopy photosynthesis. Plant Cell Environ, 2004, 27: 155-65. DOI:10.1046/j.1365-3040.2004.01142.x |
| [77] |
Iniguez C, Aguilo-Nicolau P, Galmes J. Improving photosynthesis through the enhancement of Rubisco carboxylation capacity. Biochem Soc Trans, 2021, 49: 2007-19. DOI:10.1042/BST20201056 |
| [78] |
Guo S, Zhou Y, Shen Q, et al. Effect of ammonium and nitrate nutrition on some physiological processes in higher plants-growth, photosynthesis, photorespiration, and water relations. Plant Biol, 2007, 9: 21-9. DOI:10.1055/s-2006-924541 |
| [79] |
Rachmilevitch S, Cousins AB, Bloom AJ. Nitrate assimilation in plant shoots depends on photorespiration. Proc Natl Acad Sci U S A, 2004, 101: 11506-10. DOI:10.1073/pnas.0404388101 |
| [80] |
Searles PS, Bloom AJ. Nitrate photo-assimilation in tomato leaves under short-term exposure to elevated carbon dioxide and low oxygen. Plant Cell Environ, 2003, 26: 1247-55. DOI:10.1046/j.1365-3040.2003.01047.x |
| [81] |
Sahrawy M, Avila C, Chueca A, et al. Increased sucrose level and altered nitrogen metabolism in Arabidopsis thaliana transgenic plants expressing antisense chloroplastic fructose-1, 6-bisphosphatase. J Exp Bot, 2004, 55: 2495-503. DOI:10.1093/jxb/erh257 |
| [82] |
Paul MJ, Driscoll SP, Andralojc PJ, et al. Decrease of phosphoribulokinase activity by antisense RNA in transgenic tobacco: definition of the light environment under which phosphoribulokinase is not in large excess. Planta, 2000, 211: 112-9. DOI:10.1007/s004250000269 |
| [83] |
Zhu GH, Jensen RG. Fallover of ribulose 1, 5-bisphosphate carboxylase/oxygenase activity. Plant Physiol, 1991, 97: 1354-8. DOI:10.1104/pp.97.4.1354 |
| [84] |
Jensen RG. Activation of Rubisco regulates photosynthesis at high temperature and CO2. Proc Natl Acad Sci U S A, 2000, 97: 12937-8. DOI:10.1073/pnas.97.24.12937 |
| [85] |
Bracher A, Whitney SM, Hartl FU, et al. Biogenesis and metabolic maintenance of Rubisco. Annu Rev Plant Biol, 2017, 68: 29-60. DOI:10.1146/annurev-arplant-043015-111633 |
| [86] |
Prywes N, Phillips NR, Tuck OT, et al. Rubisco function, evolution, and engineering. Annu Rev Biochem, 2023, 92: 385-410. DOI:10.1146/annurev-biochem-040320-101244 |
| [87] |
Tan BC, Lim YS, Lau SE. Proteomics in commercial crops: an overview. J Proteomics, 2017, 169: 176-88. DOI:10.1016/j.jprot.2017.05.018 |
| [88] |
Houtz RL, Magnani R, Nayak NR, et al. Co- and post-translational modifications in Rubisco: unanswered questions. J Exp Bot, 2008, 59: 1635-45. |
| [89] |
Lehtimaki N, Koskela MM, Mulo P. Posttranslational modifications of chloroplast proteins: an emerging field. Plant Physio, 2015, 168: 768-75. DOI:10.1104/pp.15.00117 |
| [90] |
Matiolli CC, Soares RC, Alves HLS, et al. Turning the knobs: the impact of post-translational modifications on carbon metabolism. Front Plant Sci, 2022, 12: 781508. DOI:10.3389/fpls.2021.781508 |
| [91] |
Amaral J, Lobo AKM, Carmo-Silva E. Regulation of Rubisco activity in crops. New phytol, 2024, 241: 35-51. DOI:10.1111/nph.19369 |
| [92] |
Gao ZF, Shen Z, Chao Q, et al. Large-scale proteomic and phosphoproteomic analyses of maize seedling leaves during de-etiolation. Genom Proteom Bioinf, 2020, 18: 397-414. DOI:10.1016/j.gpb.2020.12.004 |
| [93] |
Gao ZF, Yang X, Mei Y, et al. A dynamic phosphoproteomic analysis provides insight into the C4 plant maize (Zea mays L.) response to natural diurnal changes. Plant J, 2023, 113: 291-307. DOI:10.1111/tpj.16047 |
| [94] |
Gao X, Hong H, Li WC, et al. Downregulation of Rubisco activity by non-enzymatic acetylation of RbcL. Mol Plant, 2016, 9: 1018-27. DOI:10.1016/j.molp.2016.03.012 |
| [95] |
Yan Z, Shen Z, Gao ZF, et al. A comprehensive analysis of the lysine acetylome reveals diverse functions of acetylated proteins during de-etiolation in Zea mays. J Plant Physiol, 2020, 248: 153158. DOI:10.1016/j.jplph.2020.153158 |
| [96] |
Hartl M, Fuessl M, Boersema PJ, et al. Lysine acetylome profiling uncovers novel histone deacetylase substrate proteins in Arabidopsis. Mol Syst Biol, 2017, 13: 949. DOI:10.15252/msb.20177819 |
| [97] |
Koh J, Chen G, Yoo MJ, et al. Comparative proteomic analysis of Brassica napus in response to drought stress. J Proteome Res, 2015, 14: 3068-81. DOI:10.1021/pr501323d |
| [98] |
Lv DW, Zhu GR, Zhu D, et al. Proteomic and phosphoproteomic analysis reveals the response and defense mechanism in leaves of diploid wheat T. monococcum under salt stress and recovery. J Proteomics, 2016, 143: 93-105. DOI:10.1016/j.jprot.2016.04.013 |
| [99] |
Qiu QS, Huber JL, Booker FL, et al. Increased protein carbonylation in leaves of Arabidopsis and soybean in response to elevated CO2. Photosynthesis Res, 2008, 97: 155-66. DOI:10.1007/s11120-008-9310-5 |
| [100] |
Wang JC, Ma ZK, Li CD, et al. Dynamic responses of barley root succinyl-proteome to short-term phosphate starvation and recovery. Front Plant Sci, 2021, 12: 649147. DOI:10.3389/fpls.2021.649147 |
| [101] |
Wang LM, Jin X, Li QB, et al. Comparative proteomics reveals that phosphorylation of β carbonic anhydrase 1 might be important for adaptation to drought stress in Brassica napus. Sci Rep, 2016, 6: 39024. DOI:10.1038/srep39024 |
| [102] |
Chen XH, Zhang WF, Zhang BQ, et al. Phosphoproteins regulated by heat stress in rice leaves. Proteome Sci, 2011, 9: 37. DOI:10.1186/1477-5956-9-37 |
| [103] |
Liu ZB, Song JS, Miao W, et al. Comprehensive proteome and lysine acetylome analysis reveals the widespread involvement of acetylation in cold resistance of pepper (Capsicum annuum L.). Front Plant Sci, 2021, 12: 730489. DOI:10.3389/fpls.2021.730489 |
| [104] |
Wang XY, Liu Y, Li HX, et al. SlSNAT2, a chloroplast-localized acetyltransferase, is involved in Rubisco lysine acetylation and negatively regulates drought stress tolerance in tomato. Environ Exp Bot, 2022, 201: 105003. DOI:10.1016/j.envexpbot.2022.105003 |
| [105] |
Eckardt NA, Pell EJ. Oxidative modification of Rubisco from potato foliage response to ozone. Plant Physiol Biochem, 1995, 33: 273-82. |
| [106] |
Junqua M, Biolley JP, Pie S, et al. In vivo occurrence of carbonyl residues in Phaseolus vulgaris proteins as a direct consequence of a chronic ozone stress. Plant Physiol Biochem, 2000, 38: 853-61. DOI:10.1016/S0981-9428(00)01193-1 |
| [107] |
Romero-Puertas MC, Palma JM, Gómez M, et al. Cadmium causes the oxidative modification of proteins in pea plants. Plant Cell Environ, 2002, 25: 677-86. DOI:10.1046/j.1365-3040.2002.00850.x |
| [108] |
Orr DJ, Robijns AKJ, Baker CR, et al. Dynamics of Rubisco regulation by sugar phosphate derivatives and their phosphatases. J Exp Bot, 2023, 74: 581-90. DOI:10.1093/jxb/erac386 |
| [109] |
Parry MAJ, Keys AJ, Madgwick PJ, et al. Rubisco regulation: a role for inhibitors. J Exp Bot, 2008, 59: 1569-80. |
| [110] |
周昱婕, 李霞, 陈根云, 等. 植物RuBisCO研究进展. 中国科学: 生命科学, 2023, 53: 1213-29. |
| [111] |
Jean-Christophe D, Palmroth S, Ward E, et al. Acclimation of leaf hydraulic conductance and stomatal conductance of Pinus taeda (loblolly pine) to long-term growth in elevated CO2 (free-air CO2 enrichment) and N-fertilization. Plant Cell Environ, 2010, 32: 1500-12. |
| [112] |
Levine LH, Richards JT, Wheeler RM. Super-elevated CO2 interferes with stomatal response to ABA and night closure in soybean (Glycine max). J Plant Physiol, 2009, 166: 903-13. DOI:10.1016/j.jplph.2008.11.006 |
| [113] |
Aranjuelo I, Irigoyen JJ, Nogués S, et al. Elevated CO2 and water-availability effect on gas exchange and nodule development in N2-fixing alfalfa plants. Environl Exp Bot, 2009, 65: 18-26. DOI:10.1016/j.envexpbot.2008.06.006 |
| [114] |
卢从明, 张其德, 匡廷云, 等. 水分胁迫抑制水稻光合作用的机理. 作物学报, 1994, 5: 601-6. DOI:10.3321/j.issn:0496-3490.1994.05.014 |
| [115] |
张其德, 卢从明. 大气CO2浓度升高对光合作用的影响. 植物学报, 1992, 9: 18-23. |
| [116] |
Gilman IS, Edwards EJ. Crassulacean acid metabolism. Curr Biol, 2020, 30: R57-62. DOI:10.1016/j.cub.2019.11.073 |
| [117] |
Sutter M, Laughlin TG, Sloan NB, et al. Structure of a synthetic β-carboxysome shell. Plant Physiol, 2019, 181: 1050-8. DOI:10.1104/pp.19.00885 |
| [118] |
Zhan Y, Marchand CH, Maes A, et al. Pyrenoid functions revealed by proteomics in Chlamydomonas reinhardtii. PLoS One, 2018, 13: e0185039. DOI:10.1371/journal.pone.0185039 |
| [119] |
Price GD, Badger MR, Woodger FJ, et al. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J Exp Bot, 2008, 59: 1441-61. DOI:10.1093/jxb/erm112 |
| [120] |
Sage RF. The evolution of C4 photosynthesis. New Phytol, 2004, 161: 341-70. DOI:10.1111/j.1469-8137.2004.00974.x |
| [121] |
Price GD, Pengelly JJL, Forster B, et al. The cyanobacterial CCM as a source of genes for improving photosynthetic CO2 fixation in crop species. J Exp Bot, 2013, 64: 753-68. DOI:10.1093/jxb/ers257 |
| [122] |
Schuler ML, Mantegazza O, Weber APM. Engineering C4 photosynthesis into C3 chassis in the synthetic biology age. Plant J, 2016, 87: 51-65. DOI:10.1111/tpj.13155 |
| [123] |
Sage TL, Sage RF. The functional anatomy of rice leaves: implications for refixation of photorespiratory CO2 and efforts to engineer C4 photosynthesis into rice. Plant Cell Physiol, 2009, 50: 756-72. DOI:10.1093/pcp/pcp033 |
| [124] |
Lemaire SD, Collin V, Keryer E, et al. Chlamydomonas reinhardtii: a model organism for the study of the thioredoxin family. Plant Physiol Biochem, 2003, 41: 513-21. DOI:10.1016/S0981-9428(03)00079-2 |
| [125] |
Buchanan BB, Balmer Y. Redox regulation: a broadening horizon. Annu Rev Plant Biol, 2005, 56: 187-220. DOI:10.1146/annurev.arplant.56.032604.144246 |
| [126] |
Graciet E, Gans P, Wedel N, et al. The small protein CP12: a protein linker for supramolecular complex assembly. Biochemistry, 2003, 42: 8163-70. DOI:10.1021/bi034474x |
| [127] |
Marri L, Trost P, Trivelli X, et al. Spontaneous assembly of photosynthetic supramolecular complexes as mediated by the intrinsically unstructured protein CP12. J Biol Chem, 2008, 283: 1831-8. DOI:10.1074/jbc.M705650200 |
| [128] |
Marri L, Trost P, Pupillo P, et al. Reconstitution and properties of the recombinant glyceraldehyde-3-phosphate dehydrogenase/CP12/phosphoribulokinase supramolecular complex of Arabidopsis. Plant Physiol, 2005, 139: 1433-43. DOI:10.1104/pp.105.068445 |
| [129] |
Wedel N, Soll J. Evolutionary conserved light regulation of Calvin cycle activity by NADPH-mediated reversible phosphoribulokinase/CP12/glyceraldehyde-3-phosphate dehydrogenase complex dissociation. Proc Natl Acad Sci U S A, 1998, 95: 9699-704. DOI:10.1073/pnas.95.16.9699 |
| [130] |
McFarlane CR, Shah NR, Kabasakal BV, et al. Structural basis of light-induced redox regulation in the Calvin-Benson cycle in cyanobacteria. Proc Natl Acad Sci U S A, 2019, 116: 20984-90. DOI:10.1073/pnas.1906722116 |
| [131] |
Yu AL, Xie Y, Pan XW, et al. Photosynthetic phosphoribulokinase structures: enzymatic mechanisms and the redox regulation of the Calvin-Benson-Bassham cycle. Plant Cell, 2020, 32: 1556-73. DOI:10.1105/tpc.19.00642 |
| [132] |
Lopez-Calcagno PE, Abuzaid AO, Lawson T, et al. Arabidopsis CP12 mutants have reduced levels of phosphoribulokinase and impaired function of the Calvin-Benson cycle. J Exp Bot, 2017, 68: 2285-98. DOI:10.1093/jxb/erx084 |
| [133] |
Marri L, Zaffagnini M, Collin V, et al. Prompt and easy activation by specific thioredoxins of Calvin cycle enzymes of Arabidopsis thaliana associated in the GAPDH/CP12/PRK supramolecular complex. Mol Plant, 2009, 2: 259-69. DOI:10.1093/mp/ssn061 |
| [134] |
Nikkanen L, Rintamaki E. Chloroplast thioredoxin systems dynamically regulate photosynthesis in plants. Biochem J, 2019, 476: 1159-72. DOI:10.1042/BCJ20180707 |
| [135] |
Long SP, Ort DR. More than taking the heat: crops and global change. Curr Opin Plant Biol, 2010, 13: 241-8. |
| [136] |
Kobza J, Edwards GE. Influences of leaf temperature on photosynthetic carbon metabolism in wheat. Plant Physiol, 1987, 83: 69-74. DOI:10.1104/pp.83.1.69 |
| [137] |
Haldimann P, Feller U. Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1, 5-bisphosphate carboxylase/oxygenase. Plant Cell Environ, 2004, 27: 1169-83. DOI:10.1111/j.1365-3040.2004.01222.x |
| [138] |
von Caemmerer S. Rubisco carboxylase/oxygenase: from the enzyme to the globe: a gas exchange perspective. J Plant Physiol, 2020, 252: 153240. DOI:10.1016/j.jplph.2020.153240 |
| [139] |
Sharkey TD. Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, Rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ, 2005, 28: 269-77. DOI:10.1111/j.1365-3040.2005.01324.x |
| [140] |
Muthuramalingam M, Matros A, Scheibe R, et al. The hydrogen peroxide-sensitive proteome of the chloroplast in vitro and in vivo. Front Plant Sci, 2013, 4: 54. |
| [141] |
Chen JH, Tang M, Jin XQ, et al. Regulation of Calvin-Benson cycle enzymes under high temperature stress. aBIOTECH, 2022, 3: 65-77. DOI:10.1007/s42994-022-00068-3 |
| [142] |
Sun C, Lei Y, Li BS, et al. Precise integration of large DNA sequences in plant genomes using PrimeRoot editors. Nat Biotechnol, 2024, 42: 316-27. DOI:10.1038/s41587-023-01769-w |
| [143] |
Zong Y, Liu Y, Xue C, et al. An engineered prime editor with enhanced editing efficiency in plants. Nat Biotechnol, 2022, 40: 1394-402. DOI:10.1038/s41587-022-01254-w |
| [144] |
Borkakoti N, Thornton JM. AlphaFold2 protein structure prediction: implications for drug discovery. Curr Opin Struct Biol, 2023, 78: 102526. DOI:10.1016/j.sbi.2022.102526 |
| [145] |
Edwards C. AlphaFold spreads through protein science. Commum Acm, 2023, 66: 10-2. |
| [146] |
Zhao MX, Jiang YL, He YX, et al. Structural basis for the allosteric control of the global transcription factor NtcA by the nitrogen starvation signal 2-oxoglutarate. Proc Natl Acad Sci U S A, 2010, 107: 12487-92. DOI:10.1073/pnas.1001556107 |
 2024, Vol. 36
2024, Vol. 36 

 刘翠敏,2011年受聘于中国科学院遗传与发育生物学研究所,全职回国工作,2012年入选中国科学院“百人计划”。回国后的主要研究领域是植物光合作用碳代谢与蛋白质稳态调控,该领域研究是植物生理与生物化学学科经久不衰的热点与难点,是决定作物生物量与产量的重要决定因素之一。在前期的工作基础上,继续筛选高效碳固定关键酶Rubisco。迄今,申请人课题组已经解析了Rubisco生物合成分子伴侣Cpn60、负责Rubisco降解的蛋白酶Clp复合体的结构与功能,并揭示了Rubisco生物合成的关键调控因子,取得了阶段性的原创性成果。在国外学习工作期间以及回国工作以来,申请人在分子伴侣调控叶绿体蛋白质合成研究领域取得了系列重要进展,先后在Nature、Nature Plants、New Phytologist、BMC Biology、Molecular Plant和The Plant Journal等学术期刊上发表第一作者和通讯作者论文15篇,在Plant Cell、Nature Communications 和Plant Physiology 等期刊发表合作论文5篇。上述论文在植物发育生物学和基因组学领域得到广泛重视和认可,应邀在Frontiers in Molecular Biosciences写综述文章。多次应邀在国内外学术会议作报告,为多家国际学术刊物审稿,并多次参与国家自然科学基金委、科技部项目评审。承担的重大科研任务包括:中国科学院“百人计划”项目、转基因生物新品种培育科技重大专项、“科技创新2030”项目、中国科学院战略性先导科技专项(A类)、国家重点研发计划、国家自然科学基金项目等
刘翠敏,2011年受聘于中国科学院遗传与发育生物学研究所,全职回国工作,2012年入选中国科学院“百人计划”。回国后的主要研究领域是植物光合作用碳代谢与蛋白质稳态调控,该领域研究是植物生理与生物化学学科经久不衰的热点与难点,是决定作物生物量与产量的重要决定因素之一。在前期的工作基础上,继续筛选高效碳固定关键酶Rubisco。迄今,申请人课题组已经解析了Rubisco生物合成分子伴侣Cpn60、负责Rubisco降解的蛋白酶Clp复合体的结构与功能,并揭示了Rubisco生物合成的关键调控因子,取得了阶段性的原创性成果。在国外学习工作期间以及回国工作以来,申请人在分子伴侣调控叶绿体蛋白质合成研究领域取得了系列重要进展,先后在Nature、Nature Plants、New Phytologist、BMC Biology、Molecular Plant和The Plant Journal等学术期刊上发表第一作者和通讯作者论文15篇,在Plant Cell、Nature Communications 和Plant Physiology 等期刊发表合作论文5篇。上述论文在植物发育生物学和基因组学领域得到广泛重视和认可,应邀在Frontiers in Molecular Biosciences写综述文章。多次应邀在国内外学术会议作报告,为多家国际学术刊物审稿,并多次参与国家自然科学基金委、科技部项目评审。承担的重大科研任务包括:中国科学院“百人计划”项目、转基因生物新品种培育科技重大专项、“科技创新2030”项目、中国科学院战略性先导科技专项(A类)、国家重点研发计划、国家自然科学基金项目等