自工业革命以来,随着化石燃料的大量使用,大气中的二氧化碳浓度日益增加,导致恶劣天气频繁发生[1]。碳汇(carbon sink)和清洁能源开发是减少大气中二氧化碳浓度和实现碳中和(carbon neutrality)目标的两条关键途径[2-5]。藻类光合制氢是其中的一项重要技术,它指的是藻类细胞利用太阳光和水,在氢化酶的催化下,生产氢气的过程[6]。这一过程主要包括两条途径:一条是依赖于光系统Ⅱ的途径(图 1),约占总量的80%左右;另一条是不依赖于光系统Ⅱ的途径[7-10]。依赖于光系统Ⅱ的途径不仅有助于二氧化碳的固定和碳汇的形成,还通过生产清洁能源,为实现碳中和目标提供有力支持。
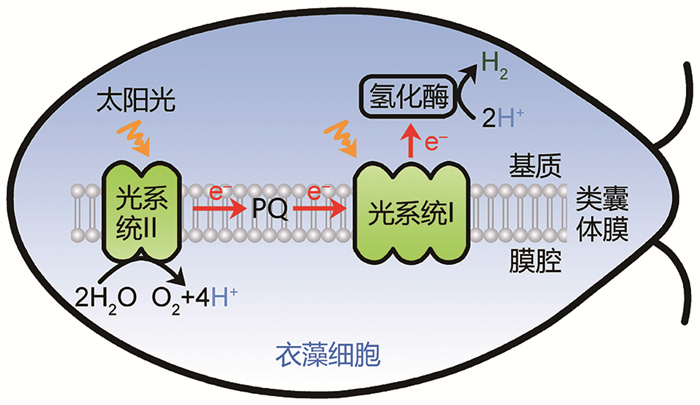
|
图 1 藻类利用光能分解水制氢过程示意图 |
1942年,Gaffron和Rubin[11]在将藻类细胞从暗适应条件移到光照下时,首次观测到藻类的光合放氢现象。然而,这一过程仅持续了几分钟,主要原因是光合作用释放的氧气导致氢化酶失活[12–14]。在过去的八十年中,通过采用各种策略[15-21],在光合作用过程中创造厌氧环境和激活氢化酶,科学家们成功地延长了藻类光合制氢的持续时间,从最初的几分钟延长至127天[22]。我国科学家在藻类光合制氢领域的研究起步较晚,直到1985年,上海植物生理生态研究所的陈因和方大惟开启了丝状蓝藻鱼腥藻7120 (Anabaena sp. strain PCC 7120)的光合制氢研究[23]。
自1985年起,我国科学家在藻类光合制氢领域取得了显著的研究进展,尤其是在研发除氧剂和细胞聚集体(cell aggregates)诱导藻类光合制氢方面。本文对我国藻类光合制氢领域的研究进展、当前面临的挑战和未来的发展方向进行全面综述与讨论,希望为研究生和科研人员提供有益的信息,促进我国藻类光合制氢的发展。
1 我国在藻类光合制氢领域的主要研究进展众所周知,藻类在光合制氢过程中释放的氧气会引发氢化酶失活,从而导致制氢过程终止。在过去的近四十年里,我国科学家通过开发多种方法,包括降低藻类光系统Ⅱ放氧活性的策略[19-20, 24-26]和清除藻类光系统Ⅱ释放的氧气的策略[18, 27-28](图 2),以创造厌氧环境,从而激活和维持氢化酶的活性,成功实现了持续光合制氢的目标。
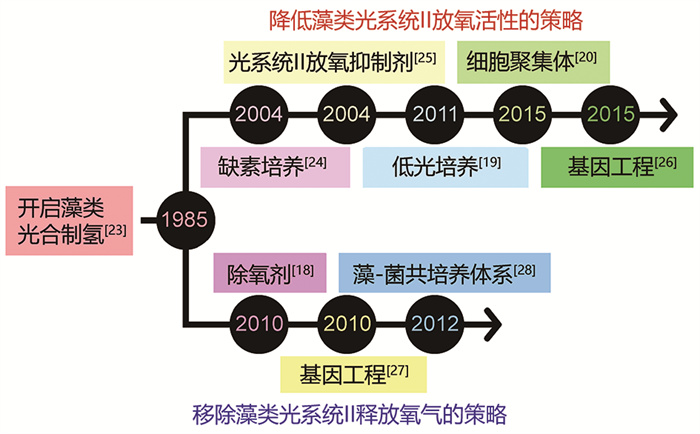
|
图 2 我国藻类光合制氢重大事件的时间线 |
在光照条件下,藻类细胞通过光系统Ⅱ分解水,产生电子,并释放出氧气[6]。这里的电子是藻类光合制氢的主要来源[6],然而,释放的氧气会抑制氢化酶活性[12-14],导致制氢过程终止。为了实现藻类持续进行光合制氢,我国科学家采用或开发了多种策略,包括降低藻类光系统Ⅱ活性的方法[19-20, 24-26](图 2),然后通过呼吸作用消耗氧气,从而建立厌氧条件,以激活氢化酶。这些方法旨在实现持续光合制氢的目标。
1.1.1 缺素培养当淡水绿藻莱茵衣藻(Chlamydomonas reinhardtii)被转移到缺硫的培养基中时,光系统Ⅱ的放氧活性受到显著抑制,但细胞呼吸的耗氧活性基本上没有受到影响。经过大约24 h,细胞进入厌氧环境,激活氢化酶,从而启动藻类光合制氢的过程[15]。类似的藻类光合制氢机制也发生在其他缺乏特定元素的培养条件下,如缺氮的培养[29]、缺磷的培养[30-31]以及缺镁的培养[32]。
在这一研究背景下,我国科学家在多个层面进行延伸和改进。具体而言,中国科学院大连化学物理研究所的张卫研究团队[24]和大连民族大学的冉春秋研究团队[33]将缺硫培养策略应用于海水绿藻亚心型扁藻(Platymonas subcordiformis)光合制氢的研究中。同时,中国科学院海洋研究所的刘建国研究团队采用缺硫或缺氮的培养策略,将其应用于从自然水体中筛选和鉴定的28种微藻中,包括13种淡水绿藻、12种海水绿藻和3种蓝藻[34]。随后,通过缺硫、缺氮和(或)缺磷的不同组合培养,他们发现这种方法可以提高小球藻的光合制氢水平[35-36]。值得注意的是,在这些不同组合中,缺氮培养导致细胞呼吸增加,从而提升不依赖光系统Ⅱ的光合制氢水平[37]。
1.1.2 光系统Ⅱ放氧抑制剂二氯苯基二甲脲(3-(3, 4-dichlorophenyl)-1, 1-dimethylurea, DCMU)是一种光系统Ⅱ放氧抑制剂,其作用是阻断光系统Ⅱ内QA到QB之间的电子传递[38]。值得注意的是,尽管DCMU抑制了光系统Ⅱ的放氧活性,但并不影响细胞呼吸的耗氧活性。这导致细胞进入厌氧状态,激活氢化酶,从而实现藻类持续光合制氢[39-41]。在相关研究中,我国科学家发现小球藻的光合制氢水平与向细胞添加DCMU的时间密切相关[42]。
羰基氰化物间氯苯腙(carbonyl cyanide m- chlorophenylhydrazone, CCCP)是另一种光系统Ⅱ放氧抑制剂,它能阻止ATP合成以及质子梯度驱动的光系统Ⅱ电子传递[43-44]。与DCMU创造厌氧环境的机制类似,它会激活氢化酶,促进蓝藻进行持续光合制氢过程[45]。我国科学家的研究结果表明,添加CCCP能够诱导多种海水绿藻实现持续光合制氢[25, 46-47]。这种持续制氢的过程与光系统Ⅱ的活性[48]和细胞呼吸的活性[49]密切相关。
1.1.3 低光培养当光照强度较低时,光系统Ⅱ释放氧气的数量会显著减少,但并不会影响细胞呼吸的氧气消耗活性。在这种情况下会形成一种厌氧环境,激活氢化酶,从而使藻类能够持续光合制氢[19, 50-51]。中国科学院青岛能源与过程研究所的郭荣波研究团队首次成功地利用低光条件培养藻类细胞,并诱导其进行持续光合制氢[19]。
1.1.4 细胞聚集体在高光条件下,分散的小球藻细胞通过光合作用释放氧气。然而,本研究团队与浙江大学唐睿康研究团队合作,发现在小球藻细胞聚集体的核心区域,由于呼吸作用消耗的氧气量超过光合作用释放的氧气量,导致产生厌氧区域,并开始发挥光合制氢功能[20](图 3A)。因此,细胞聚集体的核心区域实现了从光合放氧向光合制氢的转变。需要注意的是,在细胞聚集体的外层区域,仍然进行光合作用并释放氧气[20](图 3A)。通过在细胞聚集体中分别添加呼吸作用底物二甲基亚砜[52]和耗氧细菌大肠杆菌[53],可以扩大厌氧区域的范围,使小球藻聚集体的光合制氢效率从每升藻细胞生产19 mL氢气分别提高到31 mL [52]和51 mL [53]。尽管我国在藻类光合制氢方面起步较晚,但在利用细胞聚集体诱导藻类光合制氢方面却具有明显的国际竞争优势。
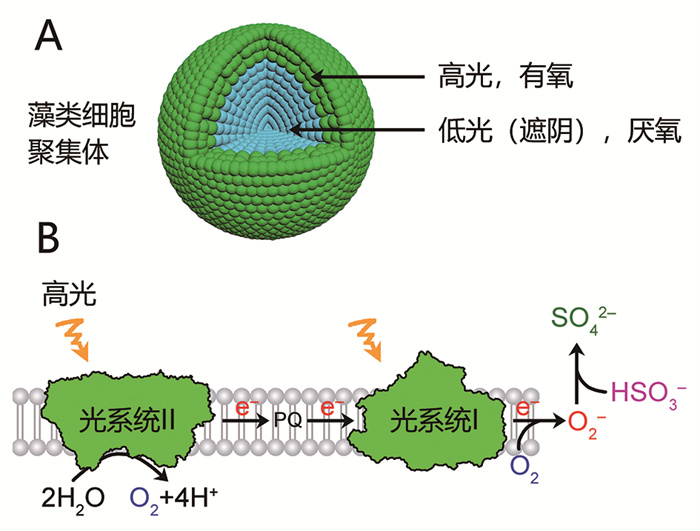
|
图 3 细胞聚集体(A)和NaHSO3处理(B)创造藻类厌氧环境的机制示意图 |
通过基因工程手段删除或下调光系统Ⅱ的核心组分,可以减少藻类光合作用释放的氧气量。随后,通过呼吸作用构建厌氧环境,激活氢化酶,从而启动藻类的光合制氢过程,并提高光合制氢的水平[16, 54-56]。深圳大学胡章立研究团队通过构建两种不同的人工miRNA表达系统,一种是热诱导表达系统,针对光系统Ⅱ相关的OEE2基因和psbA基因进行靶向调控[26, 57];另一种是蓝光诱导表达系统,针对psbA基因进行靶向调控[58]。这些系统的建立成功降低光系统Ⅱ的组装水平和活性,从而创造厌氧环境,提高莱茵衣藻光合制氢的效率[26, 57-58]。
1.2 清除藻类光系统Ⅱ释放的氧气的策略除了降低藻类光系统Ⅱ的活性外,另一个建立厌氧环境并实现藻类持续光合制氢的重要策略是移除藻类光系统Ⅱ释放的氧气[18, 27-28](图 2)。为了实现这一目标,我国科学家采用或开发了多个新技术,包括添加除氧剂、运用基因工程和使用藻-菌共培养系统。
1.2.1 除氧剂通过添加除氧剂去除藻类光系统Ⅱ释放的氧气,可以激活氢化酶,利用光系统Ⅱ光能裂解水产生的电子和质子来生产氢气。理论上,这是一个高效的诱导藻类光合制氢的策略。Na2S2O4是第一个应用于藻类光合制氢的除氧剂,然而,它的使用导致了严重的光系统Ⅱ损伤,从而导致产氢持续时间短且产量低下[59]。
本研究团队首次将除氧剂NaHSO3应用于蓝藻[18]和绿藻[60]的光合制氢过程,并揭示其除氧机制:亚硫酸氢根离子与光系统Ⅰ受体侧的超氧阴离子发生反应,从而间接地去除了光合作用释放的氧气[61](图 3B)。与Na2S2O4类似,NaHSO3的使用也会导致明显的光系统Ⅱ损伤。另外,它的使用导致细胞生长受阻,减少对铁的需求,从而导致铁积累,进而引发芬顿反应[62]。因此,缺铁可以降低铁的积累和芬顿反应的发生,从而增加光系统Ⅱ的活性和光合制氢的水平[62]。并且,通过分步添加NaHSO3并优化培养液pH,可以减轻其对光系统Ⅱ的损伤,进而提高藻类光合制氢的水平[63-64]。
除氧剂葡萄糖氧化酶利用葡萄糖为底物,在除去氧气的同时,产生过氧化氢和葡萄糖酸[65],这些产物会对藻类的活性造成损害。上海交通大学樊春海研究团队构建化学酶联级反应系统,包括葡萄糖、葡萄糖氧化酶、过氧化氢酶和氢氧化镁[66],在建立厌氧环境的同时,能够清除过氧化氢,并避免酸性环境的产生,从而延长绿藻光合制氢时间,提高制氢水平[66]。
除氧剂漆酶利用单宁酸为底物,将单宁酸的酚羟基氧化为醌的同时,还能从反应体系中去除氧气分子[67]。哈尔滨工业大学黄鑫研究团队通过在小球藻细胞表面构建多巴胺、漆酶和单宁酸组成的类三明治结构的厌氧层,实现了细胞功能从光合放氧到光合制氢的转变[67]。尽管我国在藻类光合制氢方面起步较晚,但在利用除氧剂诱导藻类光合制氢方面却具有明显的国际优势。
1.2.2 基因工程通过基因工程手段,不仅可以降低藻类释放氧气的活性,而且可以清除藻类释放的氧气,从而有效构建厌氧环境,增加氢化酶的活性,实现藻类的光合制氢过程,并提高光合制氢的水平。上海师范大学吴双秀和王全喜研究团队成功地将大豆的血红蛋白基因引入莱茵衣藻的叶绿体中,并实现异源表达。这一举措明显提升藻类对氧气的消耗能力,并显著增加光合制氢效率[27, 68]。中国科学院上海植物生理生态研究所朱新广研究团队与本研究团队合作,通过将大肠杆菌的丙酮酸氧化酶引入莱茵衣藻的叶绿体中,成功地消耗了藻类光合作用释放的氧气,从而创造了一个厌氧环境,显著增加莱茵衣藻光合制氢的效率[69]。
1.2.3 藻-菌共培养体系除了利用除氧剂和基因工程手段外,耗氧细菌也能够清除藻类光合作用释放的氧气。因此,建立藻-菌共培养系统是另一种重要策略,用于移除藻类光系统Ⅱ释放的氧气,创造厌氧环境,并激活氢化酶,从而持续光合制氢[70-71]。
我国科学家在缺硫培养的莱茵衣藻中引入不同的耗氧细菌株系,以改善光系统Ⅱ活性,增加莱茵衣藻光合制氢的水平。上海师范大学吴双秀和王全喜研究团队构建缺硫培养的莱茵衣藻与不同耗氧细菌株系的共培养体系,细菌的呼吸作用导致莱茵衣藻提前进入厌氧环境,从而改善光系统Ⅱ活性,提高藻类光合制氢水平[28, 72-75]。另外,中国石油大学(华东)葛保胜和黄方研究团队构建莱茵衣藻与中间硫单胞菌(Thiomonas intermedia)的共培养体系,除了细菌的呼吸作用导致莱茵衣藻提前进入厌氧环境外,硫单胞菌还能向莱茵衣藻提供部分硫源,共同改善光系统Ⅱ活性,提高藻类光合制氢水平[76-77]。
2 当前我国藻类光合制氢面临的主要挑战近四十年来,中国科学家在实现藻类持续光合制氢领域取得了重要进展(表 1)。然而,在建立厌氧环境时,不论是通过降低光系统Ⅱ的放氧活性还是清除光系统Ⅱ释放的氧气,都会对藻类的光合制氢电子源——光系统Ⅱ造成损伤,从而限制藻类光合制氢效率的提升,无法满足工业化应用的需求。
| 表 1 我国科学家开发的藻类光合制氢策略 |
为了应对这一挑战,中国科学家对诱导藻类持续光合制氢的策略进行优化,包括缺硫培养[24, 33, 78-81]、NaHSO3添加[18, 60-64]和细胞聚集体构建[20, 52-53]等方法。在这些优化策略中,环式电子传递突变体Δpgr5或Δpgrl1在缺硫培养条件下表现出优异的性能,能够提前进入厌氧环境并保护光系统Ⅱ活性。这使得藻类光合制氢的水平达到了目前报道的最高水平,在9天内,每升藻细胞可生产850 mL氢气[78, 82]。尽管如此,这一水平仍然无法满足实际应用的需求。这是中国乃至全球藻类光合制氢目前面临的主要挑战。
3 未来我国藻类光合制氢的发展方向我国乃至全球藻类光合制氢未来发展方向的重点是提升技术水平以满足实际应用的需求。从理论上来说,一个潜在的发展方向是寻找不会对光系统Ⅱ产生损害的除氧剂。此外,细胞聚集体具有减缓高光(藻类高效光合制氢的光照条件)对光系统Ⅱ损伤的能力[83]。因此,寻找能够引发细胞聚集体的温和除氧剂是未来我国藻类光合制氢研究的一个重要方向。
通过从自然界和突变体文库筛选并获取高效固碳的新藻株作为底盘细胞(chassis cells;是经过基因优化和设计的宿主细胞,作为合成生物学的标准化操作平台),利用合成生物学手段,构建光系统Ⅰ与氢化酶融合的超分子复合物,实现光合固碳的电子转向光合制氢[84-86]。尽管中国科学家目前尚未涉足这一领域,但合成生物学方法将成为未来我国藻类光合制氢研究的另一个关键发展方向。
4 总结尽管我国在藻类光合制氢领域起步较晚,但在研发除氧剂和细胞聚集体方面已取得明显的国际竞争优势,成为该领域的主要研究进展。本文综述了我国藻类光合制氢的研究现状、面临的挑战和未来的发展方向,旨在为研究生和科研人员提供有益的信息,推动我国藻类光合制氢事业的进一步发展,为实现碳中和目标提供有力支持。
致谢 作者衷心感谢马为民藻类光合制氢研究组的殷川名、蔡怡韵、张翠苹和项大铖在文献收集和整理方面的帮助,并感谢姚叶绘制部分图表。| [1] |
Jones MW, Peters GP, Gasser T, et al. National contributions to climate change due to historical emissions of carbon dioxide, methane, and nitrous oxide since 1850. Sci Data, 2023, 10: 155. DOI:10.1038/s41597-023-02041-1 |
| [2] |
Zhang C, Shi T, Liu J, et al. Eco-engineering approaches for ocean negative carbon emission. Sci Bull, 2022, 67: 2564-73. DOI:10.1016/j.scib.2022.11.016 |
| [3] |
Cheng Z, Joshi A, Fan LS. Chemical looping clean energy technology toward a low-carbon future. Engineering, 2023, 29: 42-4. DOI:10.1016/j.eng.2023.08.008 |
| [4] |
Xiong W, Peng Y, Ma W, et al. Microalgae-material hybrid for enhanced photosynthetic energy conversion: a promising path towards carbon neutrality. Natl Sci Rev, 2023, 10: nwad200. DOI:10.1093/nsr/nwad200 |
| [5] |
Obama B. The irreversible momentum of clean energy. Science, 2017, 355: 126-9. DOI:10.1126/science.aam6284 |
| [6] |
Wei L, Ma W. Photosynthetic H2 production: lessons from the regulation of electron transfer in microalgae. GCB Bioenergy, 2024, 16: e13118. DOI:10.1111/gcbb.13118 |
| [7] |
Chochois V, Dauvillée D, Beyly A, et al. Hydrogen production in Chlamydomonas: photosystem Ⅱ-dependent and -independent pathways differ in their requirement for starch metabolism. Plant Physiol, 2009, 151: 631-40. DOI:10.1104/pp.109.144576 |
| [8] |
Wang Y, Yang H, Zhang X, et al. Microalgal hydrogen production. Small Methods, 2020, 4: 1900514. DOI:10.1002/smtd.201900514 |
| [9] |
Volgusheva A, Styring S, Mamedov F. Increased photosystem Ⅱ stability promotes H2 production in sulfur-deprived Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A, 2013, 110: 7223-8. DOI:10.1073/pnas.1220645110 |
| [10] |
Kosourov S, Seibert M, Ghirardi ML. Effects of extracellular pH on the metabolic pathways in sulfur-deprived, H2-producing Chlamydomonas reinhardtii cultures. Plant Cell Physiol, 2003, 44: 146-55. DOI:10.1093/pcp/pcg020 |
| [11] |
Gaffron H, Rubin J. Fermentative and photochemical production of hydrogen in algae. J Gen Physiol, 1942, 26: 219-40. DOI:10.1085/jgp.26.2.219 |
| [12] |
Ghirardi ML, Togasaki RK, Seibert M. Oxygen sensitivity of algal H2 production. Appl Biochem Biotechnol, 1997, 63-65: 141-51. DOI:10.1007/BF02920420 |
| [13] |
Ghirardi ML. Implementation of photobiological H2 production: the O2 sensitivity of hydrogenases. Photosynth Res, 2015, 125: 383-93. DOI:10.1007/s11120-015-0158-1 |
| [14] |
Stripp ST, Goldet G, Brandmayr C, et al. How oxygen attacks [FeFe] hydrogenases from photosynthetic organisms. Proc Natl Acad Sci U S A, 2009, 106: 17331-36. DOI:10.1073/pnas.0905343106 |
| [15] |
Melis A, Zhang L, Forestier M, et al. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol, 2000, 122: 127-36. DOI:10.1104/pp.122.1.127 |
| [16] |
Surzycki R, Cournac L, Peltier G, et al. Potential for hydrogen production with inducible chloroplast gene expression in Chlamydomonas. Proc Natl Acad Sci U S A, 2007, 104: 17548-53. DOI:10.1073/pnas.0704205104 |
| [17] |
Bandyopadhyay A, Stöckel J, Min H, et al. High rates of photobiological H2 production by a cyanobacterium under aerobic conditions. Nat Commun, 2010, 1: 139. DOI:10.1038/ncomms1139 |
| [18] |
Wang L, Chen M, Wei L, et al. Treatment with moderate concentrations of NaHSO3 enhances photobiological H2 production in the cyanobacterium Anabaena sp. strain PCC 7120. Int J Hydrogen Energy, 2010, 35: 12777-83. DOI:10.1016/j.ijhydene.2010.08.115 |
| [19] |
Wang H, Fan X, Zhang Y, et al. Sustained photo-hydrogen production by Chlorella pyrenoidosa without sulfur depletion. Biotechnol Lett, 2011, 33: 1345-50. DOI:10.1007/s10529-011-0584-x |
| [20] |
Xiong W, Zhao X, Zhu G, et al. Silicification-induced cell aggregation for the sustainable production of H2 under aerobic conditions. Angew Chem Int Engl, 2015, 54: 11961-5. DOI:10.1002/anie.201504634 |
| [21] |
Kosourov S, Jokel M, Aro EM, et al. A new approach for sustained and efficient H2 photoproduction by Chlamydomonas reinhardtii. Energy Environ Sci, 2018, 11: 1431-6. DOI:10.1039/C8EE00054A |
| [22] |
Oncel S, Vardar-Sukan F. Photo-bioproduction of hydrogen by Chlamydomonas reinhardtii using a semi-continuous process regime. Int J Hydrogen Energy, 2009, 34: 7592-602. DOI:10.1016/j.ijhydene.2009.07.027 |
| [23] |
陈因, 方大惟. 蓝藻(Anabaena 7120)的光合放氢和参与放氢的酶. 植物生理学报, 1985, 11: 33-41. |
| [24] |
Guan Y, Deng M, Yu X, et al. Two-stage photo-biological production of hydrogen by marine green alga Platymonas subcordiformis. Biochem Eng J, 2004, 19: 69-73. DOI:10.1016/j.bej.2003.10.006 |
| [25] |
Guan Y, Zhang W, Deng M, et al. Significant enhancement of photobiological H2 evolution by carbonylcyanide m-chlorophenylhydrazone in the marine green alga Platymonas subcordiformis. Biotechnol Lett, 2004, 26: 1031. DOI:10.1023/B:BILE.0000032961.71564.00 |
| [26] |
Li H, Zhang L, Shu L, et al. Sustainable photosynthetic H2-production mediated by artificial miRNA silencing of OEE2 gene in green alga Chlamydomonas reinhardtii. Int J Hydrogen Energy, 2015, 40: 5609-16. DOI:10.1016/j.ijhydene.2015.02.073 |
| [27] |
Wu S, Yan G, Xu L, et al. Improvement of hydrogen production with expression of lba gene in chloroplast of Chlamydomonas reinhardtii. Int J Hydrogen Energy, 2010, 35: 13419-26. DOI:10.1016/j.ijhydene.2009.11.118 |
| [28] |
Wu S, Li X, Yu J, et al. Increased hydrogen production in co-culture of Chlamydomonas reinhardtii and Bradyrhizobium japonicum. Bioresour Technol, 2012, 123: 184-8. DOI:10.1016/j.biortech.2012.07.055 |
| [29] |
Philipps G, Happe T, Hemschemeier A. Nitrogen deprivation results in photosynthetic hydrogen production in Chlamydomonas reinhardtii. Planta, 2012, 235: 729-45. DOI:10.1007/s00425-011-1537-2 |
| [30] |
Batyrova KA, Tsygankov AA, Kosourov SN. Sustained hydrogen photoproduction by phosphorus-deprived Chlamydomonas reinhardtii cultures. Int J Hydrogen Energy, 2012, 37: 8834-9. DOI:10.1016/j.ijhydene.2012.01.068 |
| [31] |
Batyrova K, Gavrisheva A, Ivanova E, et al. Sustainable hydrogen photoproduction by phosphorus-deprived marine green microalgae Chlorella sp. Int J Mol Sci, 2015, 16: 2705-16. DOI:10.3390/ijms16022705 |
| [32] |
Volgusheva A, Kukarskikh G, Krendeleva T, et al. Hydrogen photoproduction in green algae Chlamydomonas reinhardtii under magnesium deprivation. RSC Adv, 2015, 5: 5633-7. DOI:10.1039/C4RA12710B |
| [33] |
Ran C, Zhang F, Sun H, et al. Effect of culture medium on hydrogen production by sulfur-deprived marine green algae Platymonas subcordiformis. Biotechnol Bioprocess Eng, 2009, 14: 835-41. DOI:10.1007/s12257-008-0287-x |
| [34] |
He M, Li L, Liu J. Isolation of wild microalgae from natural water bodies for high hydrogen producing strains. Int J Hydrogen Energy, 2012, 37: 4046-56. DOI:10.1016/j.ijhydene.2011.11.089 |
| [35] |
Zhang L, He M, Liu J. The enhancement mechanism of hydrogen photoproduction in Chlorella protothecoides under nitrogen limitation and sulfur deprivation. Int J Hydrogen Energy, 2014, 39: 8969-76. DOI:10.1016/j.ijhydene.2014.04.045 |
| [36] |
Pongpadung P, Zhang LT, Sathasivam R, et al. Stimulation of hydrogen photoproduction in Chlorella sorokiniana subjected to simultaneous nitrogen limitation and sulfur- and/or phosphorus-deprivation. J Pure Appl Microbiol, 2018, 12: 1719-27. DOI:10.22207/JPAM.12.4.04 |
| [37] |
Li L, Zhang L, Gong F, et al. Transcriptomic analysis of hydrogen photoproduction in Chlorella pyrenoidosa under nitrogen deprivation. Algal Res, 2020, 47: 101827. DOI:10.1016/j.algal.2020.101827 |
| [38] |
Metz JG, Pakrasi HB, Seibert M, et al. Evidence for a dual function of the herbicide-binding D1 protein in photosystem Ⅱ. FEBS Lett, 1986, 205: 269-74. DOI:10.1016/0014-5793(86)80911-5 |
| [39] |
Abeles FB. Cell-free hydrogenase from Chlamydomonas. Plant Physiol, 1964, 39: 169-76. DOI:10.1104/pp.39.2.169 |
| [40] |
Rashid N, Rehman MSU, Memon S, et al. Current status, barriers and developments in biohydrogen production by microalgae. Renew Sustain Energy Rev, 2013, 22: 571-9. DOI:10.1016/j.rser.2013.01.051 |
| [41] |
Laurinavichene T, Tolstygina I, Tsygankov A. The effect of light intensity on hydrogen production by sulfur-deprived Chlamydomonas reinhardtii. J Biotechnol, 2004, 114: 143-51. DOI:10.1016/j.jbiotec.2004.05.012 |
| [42] |
Liu JZ, Ge YM, Xia SY, et al. Photoautotrophic hydrogen production by Chlorella pyrenoidosa without sulfur-deprivation. Int J Hydrogen Energy, 2016, 41: 8427-32. DOI:10.1016/j.ijhydene.2016.03.191 |
| [43] |
Samuilov VD, Barsky EL, Gubanova ON, et al. Photoreduction of silicomolybdate in chloroplasts by agents accelerating the deactivation reactions of the water-oxidizing system. FEBS Lett, 1995, 357: 55-7. DOI:10.1016/0014-5793(94)01309-O |
| [44] |
McCauley SW, Melis A, Tang GMS, et al. Protonophores induce plastoquinol oxidation and quench chloroplast fluorescence: evidence for a cyclic, proton-conducting pathway in oxygenic photosynthesis. Proc Natl Acad Sci U S A, 1987, 84: 8424-8. DOI:10.1073/pnas.84.23.8424 |
| [45] |
Abdel-Basset R, Bader KP. Physiological analyses of the hydrogen gas exchange in cyanobacteria. J Photochem Photobiol B, 1998, 43: 146-51. DOI:10.1016/S1011-1344(98)00097-9 |
| [46] |
Ran C, Yu X, Jin M, et al. Photobiological hydrogen production by marine green alga Platymonas subcordiformis under carbonylcyanide-m-chlorophenylhydrazone. J Chem Ind Eng (China), 2004, 55: 108-12. |
| [47] |
Ran CQ, Zhang W, Yu XJ, et al. Regulation of hydrogen production by uncouplering CCCP in green algae Chlamydomonas reinhardtii. Chem Res Chin Univ, 2006, 27: 62-6. |
| [48] |
Ran C, Yu X, Jin M, et al. Role of carbonyl cyanide m-chlorophenylhydrazone in enhancing photobiological hydrogen production by marine green alga Platymonas subcordiformis. Biotechnol Prog, 2006, 22: 438-43. DOI:10.1021/bp050289u |
| [49] |
Guo Z, Chen Z, Zhang W, et al. Improved hydrogen photoproduction regulated by carbonylcyanide m-chlorophenylhrazone from marine green alga Platymonas subcordiformis grown in CO2-supplemented air bubble column bioreactor. Biotechnol Lett, 2008, 30: 877-83. DOI:10.1007/s10529-008-9637-1 |
| [50] |
Jurado-Oller JL, Dubini A, Galván A, et al. Low oxygen levels contribute to improve photohydrogen production in mixotrophic non-stressed Chlamydomonas cultures. Biotechnol Biofuels, 2015, 8: 149. DOI:10.1186/s13068-015-0341-9 |
| [51] |
Scoma A, Durante L, Bertin L, et al. Acclimation to hypoxia in Chlamydomonas reinhardtii: can biophotolysis be the major trigger for long-term H2 production?. New Phytol, 2014, 204: 890-900. DOI:10.1111/nph.12964 |
| [52] |
Shu L, Xiong W, Shao C, et al. Improvement in the photobiological hydrogen production of aggregated Chlorella by dimethyl sulfoxide. ChemBioChem, 2018, 19: 669-73. DOI:10.1002/cbic.201700637 |
| [53] |
Xu Z, Wang S, Zhao C, et al. Photosynthetic hydrogen production by droplet-based microbial micro-reactors under aerobic conditions. Nat Commun, 2020, 11: 5985. DOI:10.1038/s41467-020-19823-5 |
| [54] |
Lin HD, Liu BH, Kuo TT, et al. Knockdown of PsbO leads to induction of HydA and production of photobiological H2 in the green alga Chlorella sp. DT. Bioresour Technol, 2013, 143: 154-62. DOI:10.1016/j.biortech.2013.05.101 |
| [55] |
Torzillo G, Scoma A, Faraloni C, et al. Increased hydrogen photoproduction by means of a sulfur-deprived Chlamydomonas reinhardtii D1 protein mutant. Int J Hydrogen Energy, 2009, 34: 4529-36. DOI:10.1016/j.ijhydene.2008.07.093 |
| [56] |
Makarova VV, Kosourov S, Krendeleva TE, et al. Photoproduction of hydrogen by sulfur-deprived C. reinhardtii mutants with impaired photosystem Ⅱ photochemical activity. Photosynth Res, 2007, 94: 79-89. DOI:10.1007/s11120-007-9219-4 |
| [57] |
Li H, Liu Y, Wang Y, et al. Improved photobio-H2 production regulated by artificial miRNA targeting psbA in green microalga Chlamydomonas reinhardtii. Biotechnol Biofuels, 2018, 11: 36. DOI:10.1186/s13068-018-1030-2 |
| [58] |
Wang Y, Jiang X, Hu C, et al. Optogenetic regulation of artificial microRNA improves H2 production in green alga Chlamydomonas reinhardtii. Biotechnol Biofuels, 2017, 10: 257. DOI:10.1186/s13068-017-0941-7 |
| [59] |
Pow T, Krasna AI. Photoproduction of hydrogen from water in hydrogenase-containing algae. Arch Biochem Biophys, 1979, 194: 413-21. DOI:10.1016/0003-9861(79)90635-0 |
| [60] |
Ma W, Chen M, Wang L, et al. Treatment with NaHSO3 greatly enhances photobiological H2 production in the green alga Chlamydomonas reinhardtii. Bioresour Technol, 2011, 102: 8635-8. DOI:10.1016/j.biortech.2011.03.052 |
| [61] |
Wei L, Yi J, Wang L, et al. Light intensity is important for hydrogen production in NaHSO3-treated Chlamydomonas reinhardtii. Plant Cell Physiol, 2017, 58: 451-7. |
| [62] |
Jiang Y, Sun M, Zheng M, et al. Iron deficiency suppresses the Fenton reaction and boosts photosynthetic H2 production in bisulfite-treated Chlamydomonas cells. Chem Eng J, 2024, 485: 149872. DOI:10.1016/j.cej.2024.149872 |
| [63] |
Wei L, Li X, Fan B, et al. A stepwise NaHSO3 addition mode greatly improves H2 photoproduction in Chlamydomonas reinhardtii. Front Plant Sci, 2018, 9: 1532. DOI:10.3389/fpls.2018.01532 |
| [64] |
Wei L, Fan B, Yi J, et al. Mechanistic insights into pH-dependent H2 photoproduction in bisulfte-treated Chlamydomonas cells. Biotechnol Biofuels, 2020, 13: 64. DOI:10.1186/s13068-020-01704-0 |
| [65] |
Bankar SB, Bule MV, Singhal RS, et al. Glucose oxidase -- an overview. Biotechnol Adv, 2009, 27: 489-501. DOI:10.1016/j.biotechadv.2009.04.003 |
| [66] |
Chen J, Li J, Li Q, et al. Engineering a chemoenzymatic cascade for sustainable photobiological hydrogen production with green algae. Energy Environ Sci, 2020, 13: 2064-8. DOI:10.1039/D0EE00993H |
| [67] |
Su D, Qi J, Liu X, et al. Enzyme-modulated anaerobic encapsulation of Chlorella cells allows switching from O2 to H2 production. Angew Chem Int Ed Engl, 2019, 58: 3992-5. DOI:10.1002/anie.201900255 |
| [68] |
Xu L, Wang Q, Wu S, et al. Improvement of hydrogen yield of lba-transgenic Chlamydomonas reinhardtii caused by increasing respiration and impairing photosynthesis. Int J Hydrogen Energy, 2014, 39: 13347-52. DOI:10.1016/j.ijhydene.2014.04.178 |
| [69] |
Xu FQ, Ma WM, Zhu XG. Introducing pyruvate oxidase into the chloroplast of Chlamydomonas reinhardtii increases oxygen consumption and promotes hydrogen production. Int J Hydrogen Energy, 2011, 36: 10648-54. DOI:10.1016/j.ijhydene.2011.05.130 |
| [70] |
Giri DD, Dwivedi H, Alsukaibi AKD, et al. Sustainable production of algae-bacteria granular consortia based biological hydrogen: new insights. Bioresour Technol, 2022, 352: 127036. DOI:10.1016/j.biortech.2022.127036 |
| [71] |
Fakhimi N, Gonzalez-Ballester D, Fernández E, et al. Algae-bacteria consortia as a strategy to enhance H2 production. Cells, 2020, 9: 1353. DOI:10.3390/cells9061353 |
| [72] |
Xu L, Cheng X, Wu S, et al. Co-cultivation of Chlamydomonas reinhardtii with Azotobacter chroococcum improved H2 production. Biotechnol Lett, 2017, 39: 731-8. DOI:10.1007/s10529-017-2301-x |
| [73] |
Li X, Huang S, Yu J, et al. Improvement of hydrogen production of Chlamydomonas reinhardtii by co-cultivation with isolated bacteria. Int J Hydrogen Energy, 2013, 38: 10779-87. DOI:10.1016/j.ijhydene.2013.02.102 |
| [74] |
Xu L, Cheng X, Wang Q. Effect of co-cultivation of Chlamydomonas reinhardtii with Azotobacter chroococcum on hydrogen production. Int J Hydrogen Energy, 2017, 42: 22713-9. DOI:10.1016/j.ijhydene.2017.06.223 |
| [75] |
Xu L, Li D, Wang Q, et al. Improved hydrogen production and biomass through the co-cultivation of Chlamydomonas reinhardtii and Bradyrhizobium japonicum. Int J Hydrogen Energy, 2016, 41: 9276-83. DOI:10.1016/j.ijhydene.2016.04.009 |
| [76] |
He J, Xi L, Sun X, et al. Enhanced hydrogen production through co-cultivation of Chlamydomonas reinhardtii CC-503 and a facultative autotrophic sulfide-oxidizing bacterium under sulfurated conditions. Int J Hydrogen Energy, 2018, 43: 15005-13. DOI:10.1016/j.ijhydene.2018.06.081 |
| [77] |
Ge B, He J, Zhang Q, et al. Evaluation of various sulfides for enhanced photobiological H2 production by a dual-species co-culture system of Chlamydomonas reinhardtii and Thiomonas intermedia. Proc Biochem, 2019, 82: 110-6. |
| [78] |
Chen M, Zhang J, Zhao L, et al. Loss of algal Proton Gradient Regulation 5 increases reactive oxygen species scavenging and H2 evolution. J Integr Plant Biol, 2016, 58: 943-6. |
| [79] |
Chen M, Liu P, Zhang J, et al. Photochemical characteristics of Chlamydomonas mutant hpm91 lacking proton gradient regulation 5 (PGR5) during sustained H2 photoproduction under sulfur deprivation. Int J Hydrogen Energy, 2019, 44: 31790-9. |
| [80] |
Liu P, Ye DM, Chen M, et al. Scaling-up and proteomic analysis reveals photosynthetic and metabolic insights toward prolonged H2 photoproduction in Chlamydomonas hpm91 mutant lacking proton gradient regulation 5 (PGR5). Photosynth Res, 2022, 154: 397-411. |
| [81] |
Sun Y, Chen M, Yang H, et al. Enhanced H2 photoproduction by down-regulation of ferredoxin-NADP+ reductase (FNR) in the green alga Chlamydomonas reinhardtii. Int J Hydrogen Energy, 2013, 38: 16029-37. |
| [82] |
Steinbeck J, Nikolova D, Weingarten R, et al. Deletion of proton gradient regulation 5 (PGR5) and PGR5-like 1 (PGRL1) proteins promote sustainable light-driven hydrogen production in Chlamydomonas reinhardtii due to increased PSII activity under sulfur deprivation. Front Plant Sci, 2015, 6: 892. |
| [83] |
Xiong W, Yang Z, Zhai H, et al. Alleviation of high light-induced photoinhibition in cyanobacteria by artificially conferred biosilica shells. Chem Commun, 2013, 49: 7525-7. |
| [84] |
Kanygin A, Milrad Y, Thummala C, et al. Rewiring photosynthesis: a photosystem Ⅰ-hydrogenase chimera that makes H2 in vivo. Energy Environ Sci, 2020, 13: 2903-14. |
| [85] |
Kanygin A, Smith A, Nagy V, et al. Interplay between hydrogen production and photosynthesis in a green alga expressing an active photosystem Ⅰ-hydrogenase chimera. Int J Hydrogen Energy, 2022, 47: 21969-83. |
| [86] |
Appel J, Hueren V, Boehm M, et al. Cyanobacterial in vivo solar hydrogen production using a photosystem Ⅰ-hydrogenase (PsaD-HoxYH) fusion complex. Nat Energy, 2020, 5: 458-67. |
 2024, Vol. 36
2024, Vol. 36 

 马为民,上海师范大学生命科学学院教授,博士生导师,国家重点研发计划首席科学家。2007年毕业于中国科学院上海生命科学研究院植物生态生理研究所,获得理学博士学位。同年加入上海师范大学生命科学学院,一直致力于蓝藻光合作用和藻类光合制氢的研究。主持科技部和国家自然科学基金委员会等国家级项目7项,以及中央军委科学技术委员会、教育部和上海市科学技术委员会等省部级项目8项。作为通讯作者(含共同通讯作者),在Nature Communications、Angewandte Chemie International Edition、Bioresource Technology和Chemical Engineering Journal等SCI期刊上发表学术论文60余篇。获得5项国内发明专利。现任中国藻类学会常务理事、中国光合作用专业委员会委员和上海市一碳生物技术专委会副主任
马为民,上海师范大学生命科学学院教授,博士生导师,国家重点研发计划首席科学家。2007年毕业于中国科学院上海生命科学研究院植物生态生理研究所,获得理学博士学位。同年加入上海师范大学生命科学学院,一直致力于蓝藻光合作用和藻类光合制氢的研究。主持科技部和国家自然科学基金委员会等国家级项目7项,以及中央军委科学技术委员会、教育部和上海市科学技术委员会等省部级项目8项。作为通讯作者(含共同通讯作者),在Nature Communications、Angewandte Chemie International Edition、Bioresource Technology和Chemical Engineering Journal等SCI期刊上发表学术论文60余篇。获得5项国内发明专利。现任中国藻类学会常务理事、中国光合作用专业委员会委员和上海市一碳生物技术专委会副主任