光合作用电子传递有两种不同途径:线性电子传递(linear electron transport, LET)和围绕光系统Ⅰ的环式电子传递(cyclic electron transport around photosystem Ⅰ, CET)[1-2],涉及两个光系统(光系统Ⅰ和Ⅱ,PSⅠ、PSⅡ),它们之间的电子传递由细胞色素b6f复合物(Cyt b6f)介导,耦联类囊体膜间质侧的质子跨膜向类囊体腔内转移,形成膜内外质子浓度差(ΔpH),以驱动ATP合酶合成ATP。与LET形成对照,CET将电子从PSI还原侧传递回质醌池(plastoquinone pool, PQ pool),只需要PSI光化学反应参与就可以与Cyt b6f产生ΔpH,此过程只产生ATP,不会积累NADPH。被子植物中有两种部分冗余的CET途径,这两种途径都依赖铁氧还蛋白(Fd)还原PQ。在蓝藻和C4植物中,CET主要是由叶绿体NADPH脱氢酶类(NDH)复合物介导;而在C3植物中,CET主要是由PGR5/PGRL1 (质子梯度调控蛋白5/质子梯度调节类蛋白1)介导。原核光合生物蓝藻没有细胞器,其呼吸电子传递链利用质醌代替泛醌;Fd的电子还可以通过硝酸还原酶(NAR)或亚硝酸还原酶(NIR)参与硝酸盐和亚硝酸盐的代谢过程,细胞质的代谢产物如糖原等经糖酵解进入呼吸代谢,在NDH-1的作用下传递给光合电子载体PQ,经末端氧化酶把电子交给氧分子。在厌氧条件下,Fd的电子传递经氢化酶(Hox)产生氢气[3],这些旁路电子传递途径被称为交替电子传递途径。光合电子传递途径如图 1所示。
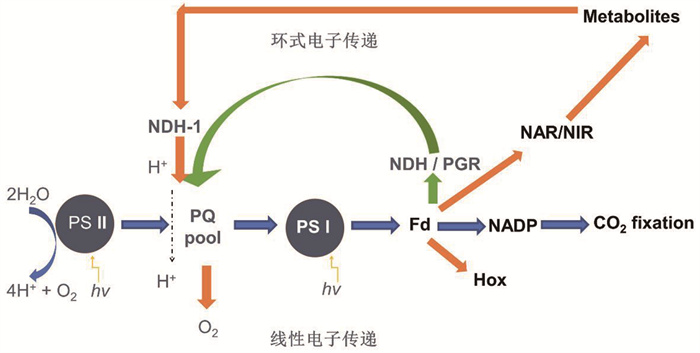
|
蓝色箭头代表线性电子传递途径;绿色箭头代表环式电子传递途径;橘色箭头代表交替电子传递途径。 图 1 光合电子传递途径示意图 |
叶绿体或蓝藻细胞的电子传递是在PSⅡ反应中心叶绿素(P680)和PSⅠ反应中心叶绿素(P700)吸收光能发生电荷分离即原初反应(光反应)后进行的。来自PSⅡ的电子还原醌池的质醌,经Cyt b6f复合体,再由可移动的电子递体质蓝素把电子带给PSⅠ使Fd还原,这种具有强还原势的还原产物将电子传递给NADP+从而形成NADPH,该途径被称为线性电子传递。当电子流通过醌池以及Cyt b6f复合体时,产生跨类囊体膜质子浓度差,推动ATP合酶合成ATP。线性电子传递在类囊体腔内进行水裂解,在外类囊体膜侧进行NADP+还原。电子还可以从PSⅠ还原侧的Fd传递回质醌池,经过叶绿体末端氧化酶或蓝藻的细胞色素c末端氧化酶将电子交给氧分子,这条途径被称为环式电子传递。环式电子传递只需要PSI光化学反应参与就可以与醌池或Cyt b6f产生ΔpH,此过程只产生ATP,不会积累NADPH。
在20世纪80年代中期,日本两个实验室分别在苔藓[4]和烟草[5]的叶绿体基因组中发现编码线粒体NADH-泛醌氧化还原酶(复合体Ⅰ)亚基的高度同源开放阅读框(ndh基因)。之后,人们开始寻找其基因产物NDH的分子实体,通过反向遗传学、蛋白质组学等手段先后鉴定出至少17个NDH亚基。NDH复合体参与CET的实验证据首先来自蓝藻的研究[6-9]。通过对蓝藻集胞藻PCC6803的NDH-B缺失突变体的P700氧化还原速率和叶绿素荧光动力学等生理生化特性进行分析,首次证明NDH不仅参与呼吸电子传递而且参与CET。随后,对高等植物叶绿体ndh基因缺失突变体的研究工作也证明NDH复合体参与CET,该途径对抗霉素A不敏感[10-13]。由于NDH位于高等植物叶绿体间质类囊体膜上,推测NDH位于PSⅠ附近进行CET的区域[10, 12-16]。根据热耗散减少可导致叶绿素荧光发射增强的原理,研究人员分离到一种缺失质子梯度调节蛋白的拟南芥突变体pgr5,发现这种类囊体膜蛋白PGR5参与由Fd到质醌的CET途径,该途径与跨膜质子梯度的建立有关,并对抗霉素A敏感[17],可能参与植物抵御强光胁迫[18]。由此推测,由PGRL1/PGR5介导的CET途径的主要功能是光破坏防御和调节NADPH/ATP的比例,而由NDH介导的CET途径的主要功能是防止间质电子受体的过度还原[19]。在蓝藻中,NDH还参与CO2吸收[20],两条途径在一定程度上互为补充,不能同时缺失[21]。通过对拟南芥pgrl1突变体的研究进一步发现,PGRL1可能与PGR5相互作用,共同调节围绕PSI的循环电子传递[22]。进一步的研究证实,PGRL1作为铁氧还蛋白-质醌还原酶(ferredoxin-plastoquinone reductase, FQR),依赖于PGR5从Fd接收电子,然后去还原PQ。这条由PGRL1/PGR5介导的循环电子传递途径对抗霉素A敏感[23]。
1 光合环式电子传递的功能综合国际上的有关研究工作,CET的功能有如下几方面(图 2):
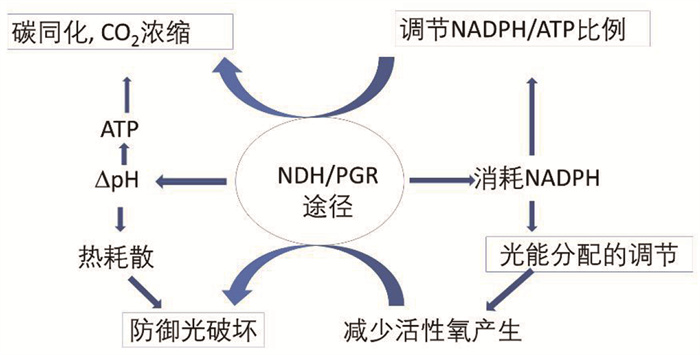
|
由NDH或PGR介导的环式电子传递消耗NADPH,能够调节不同发育时期或胁迫条件下的NADPH/ATP比例,以保障光合碳同化以及蓝藻CO2浓缩机制(CCM)的运转;同时,依赖环式电子传递产生的跨类囊体膜质子浓度差(∆pH)耦联循环光合磷酸化,产生额外ATP,用于光合碳同化和CCM,参与胁迫条件下的热耗散过程,发挥光破坏防御作用;环式电子传递最终将电子交给氧分子,使醌池相对氧化,调节激发能在两个光系统之间的分配,防止光系统的过还原,减少高温、高光胁迫条件下活性氧的产生,参与植物的光保护防御机制。 图 2 光合环式电子传递的功能 |
激发能在两个光系统之间的再分配(即状态转换)主要是调节两个光系统光激发的不平衡,也是植物防止反应中心遭受过多激发能引起的光破坏的一种防御机制。NDH通过CET参与激发能在两个光系统之间的再分配(状态转换),蓝藻NDH失活的突变体丧失状态转换的功能,被锁定在状态1。NDH介导的CET可能通过影响质醌的氧化还原状态,从而参与激发能的再分配[24]。CET和呼吸电子传递通过调节集胞藻6803藻胆体的移动,参与激发能在两个光系统的分配[25]。拟南芥的NDH途径通过影响醌池的氧化还原状态,从而参与状态转换[26]。
1.2 调节蓝藻无机碳运输蓝藻之所以能够适应水中低CO2浓度环境,是由于它们能够提高Rubisco活性部位周围的CO2浓度,弥补Rubisco对CO2亲和力低的缺陷,从而有效地同化CO2[27]。这种适应低CO2的机制被称为蓝藻CO2浓缩机制(CO2-concentrating mechanisms, CCM)。蓝藻的CCM和无机碳运输都是消耗ATP的过程。NDH的亚基NdhB所在的NDH复合体参与蓝藻的呼吸作用和CET,为CO2吸收提供所需能量[28-29]。根据蓝藻NDH不同亚基缺失突变体的研究结果,推测NDH为蓝藻CCM提供ATP,参与对CO2的吸收[20]。此外,NDH亚基的表达及其活性为低CO2浓度所诱导,推测NDH介导的CET参与蓝藻CCM[30]。通过研究NDH对高低CO2浓度的响应,提出CO2可作为信号,调控NDH的表达;同时,NDH也反过来对碳同化进行调节。CO2浓度的高低影响集胞藻6803和嗜热聚球藻NDH复合体的活性和构成:当将蓝藻细胞从高CO2浓度转至低CO2浓度时,NDH-1L活性下调,而NDH-1M上调,并且NDH亚基的表达量也增加[31]。根据功能分析结果认为,NDH-1L主要参与细胞呼吸和光异养,NDH-1MS和CO2的吸收有关,而NDH-1M参与的CET为CO2吸收提供所需能量;在ndhB缺失突变体中检测不到NDH-1L和NDH-1M复合体,但是当培养在pH 8.3的低CO2浓度条件下时,细胞能产生NDH-1S复合体并且可以利用HCO3-,这说明低CO2诱导的NDH-1S复合体不能单独承担转运CO2的功能,必须由NDH-1MS共同作用。
1.3 抵御环境胁迫强光会抑制光合作用,过多的光能甚至造成PSⅡ反应中心被破坏,而CET参与将多余的光能通过热耗散机制无害地散失,从而保护植物免受强光引起的光损伤。CET通过产生跨膜质子梯度对PSⅡ下游进行调节从而减轻光抑制。在高温条件下,缺失NDH-C、K、J亚基的烟草突变体较野生型更早地出现叶片萎蔫、茎部褐变等表型;经过进一步研究发现,其叶片中积累了过氧化氢[32-33]。另外,低浓度亚硫酸氢钠诱导的NDH途径能够减轻烟草暗光转换期间过氧化氢引起的光氧化胁迫[34-35],而光下增强的拟南芥PGR5途径发挥光破坏防御作用[36]。PGR5在拟南芥适应波动的光照条件方面也发挥关键作用[37],拟南芥的CET与光呼吸途径协同保护植物免受波动光胁迫,从而维持有效的光合作用效率[38]。这些结果都表明CET可以耗散过多的激发能,从而缓解光氧化胁迫。在番茄响应低温环境时,PGR5和NDH介导的环式电子传递都发挥光破坏防御作用[39]。研究还发现,拟南芥PGRL1通过氧化还原缓解光强变化引起的光抑制,优化光合作用[40]。叶绿体的氧化还原平衡对于光合作用的有效运转十分重要,PGR途径与硫氧还蛋白(Trx)系统共同参与维持叶绿体内的氧化还原平衡,维持光合作用活性[41]。
2 改善光合电子传递的策略 2.1 敲除竞争途径不同电子传递途径之间存在竞争关系,改善特定途径电子传递速率最为有效的方法是利用分子生物学手段,通过同源重组敲除参与竞争途径的关键基因[3]。例如,在集胞藻6803中敲除三个编码末端氧化酶的基因,可阻止电子从醌池到氧分子的传递,从而使得细胞外到铁氰化钾的电子传递速率增加24倍[42]。然而,这样大幅度的增加主要来自依赖储存代谢物的有氧呼吸的电子传递途径;而在光下,用于CO2同化的电子传递主要来源于光反应过程,即由水的光裂解产生。集胞藻中的双向氢化酶Hox从Fd接受电子,敲除竞争的Fd电子受体可以显著促进光下产氢[43]。
2.2 控制电子受体的定位电子载体的位置对于电子的有效供应以及与交替电子传递途径之间的竞争至关重要。因此,控制重要电子传递组分在超分子组织中的位置是加强特定电子传递途径的一个有希望的策略。通过基因突变,可使两个通常独立的复合体结合在一起。PSⅠ的受体侧是产生最大还原力的位点,而PSⅠ有几个亚基暴露在间质侧,有利于和其他蛋白的结合。一个成功的例子是,将集胞藻的双向氢化酶结合在其PSⅠ截短的PsaD亚基上,结果显示该突变体在厌氧条件下依赖光的产氢速率显著增强,这可能是由于电子从PSⅠ受体侧的铁硫簇直接转移到了氢化酶;该突变体还能够光自养生长,这表明氢化酶能够在PSⅠ受体侧与Fd有效地竞争电子,但不会完全阻断Fd和FNR参与的电子传递途径[44]。
2.3 融合异源电子受体β-变形杆菌的氢化酶具有较强的耐氧性,并且可能避免蓝藻光合放氧对产氢的影响。将集胞藻PSⅠ组装复合物以及从Ralstonia中分离的氢化酶和PSⅠ的PsaE亚基融合,能在体外光下高效产氢[45]。
2.4 导入或过表达PGR5、黄素二铁蛋白以加强CET将火炬松的pgr5基因在拟南芥中过表达,可加强转基因植株的环式电子传递,使其PSⅠ能够耐受强光胁迫,从而提高转基因植株在高光、干旱条件下的存活率[46]。然而,在三角褐指藻中过表达PGRL1却导致生长速率和表观PSⅡ活性显著降低[47],这可能与PGRL1不是三角褐指藻环式电子传递调控因子有关;此外,蓝藻的PGR5与PGRL1不足以调节环式电子传递[48]。
将另苔藓的黄素二铁蛋白导入PGR5 (pgr5-RNAi)和NDH (crr6突变)都缺失的水稻双突变体中,创建一条新的光合环式电子传递通路,这条途径将过剩的电子传递给氧分子,从而达到耗散过剩激发能,缓解波动光引起的PSⅠ光损伤,但又不与光合碳同化竞争电子的目的[49]。
2.5 C4植物中的CET改造在C4植物中,环式电子传递起到至关重要的作用。与C3光合作用相比,C4光合作用固定1分子CO2需要2分子ATP,多需要的ATP通常由增加的环式电子传递提供[50]。在C4植物中,PGR5依赖的和NDH依赖的两种环式电子传递都增加[51],暗示两种环式电子传递在C4光合作用中都起到重要作用。近期的研究表明,在C4光合作用中,NDH依赖的环式电子传递起到非常重要的作用。一方面,要维持高效的C4光合作用效率,维管束鞘细胞内需要维持高度氧化的状态[52];另一方面,在NDH依赖的环式电子传递缺失突变体中,NADPH: NADP大幅度提高[53]。这两个证据表明,NDH依赖的环式电子传递能够维持维管束鞘细胞相对较高的氧化态,从而维持C4光合作用的高效进行。因此,在C4光合作用中,要大力提高NDH依赖的环式电子传递能力。在C4植物黄顶菊中过表达PGR5可以增加PSⅠ下游的电子库,但不影响光合碳同化[54]。
目前对于在C4光合作用中如何上调NDH依赖的环式电子传递尚不清楚,然而大量在C3植物中的研究表明氧化还原状态可能是影响NDH表达的关键调控因子。在拟南芥中,抗坏血酸过氧化物酶(ascorbate peroxidases)的诱导表达可以有效地抑制NDH-A及NDH-D的表达[55];无论是外源施加,还是内源过表达过氧化氢,都可以诱导NDH的环式电子传递[56]。
大量证据表明,环式电子传递以及其他交替电子传递对于维持C3和C4光合作用至关重要。改善电子传递是提高作物光合效率的有效途径之一。当前,环式电子传递的调控机制还远未被揭示出来,这极大地限制了基于环式电子传递的作物光合效率的改造。将来,通过基因组学、转录组学、蛋白质组学、代谢组学、生物信息学以及网络调控模型等研究手段的有效结合,系统验证关键调控基因,有望阐明控制CET的关键调控因子,从而通过有效调控CET实现提高光能利用效率的总目标。
| [1] |
米华玲, 沈允钢. 电子传递与磷酸化[M]//陈晓亚, 薛红卫. 植物生理与分子生物学(第四版). 北京: 高等教育出版社, 2012
|
| [2] |
Joliot P, Johnson GN. Regulation of cyclic and linear electron flow in higher plants. Proc Natl Acad Sci U S A, 2011, 108: 13317-22. DOI:10.1073/pnas.1110189108 |
| [3] |
Mullineaux CW. Improving the transport of electrons[M]//Ruban A, Foyer CH, Murchie EH. Photosynthesis in action. Harvesting light, generating electrons, fixing carbon. New York: Academic Press, 2022: 161-74
|
| [4] |
Ohyama K, Fukuzawa H, Kohchi T, et al. Chloroplast gene organization deduced from complete sequence of liverwort Marchantia polymorpha chloroplast DNA. Nature, 1986, 322: 572-4. DOI:10.1038/322572a0 |
| [5] |
Shinozaki K, Ohme M, Tanaka M, et al. The complete nucleotide-sequence of the tobacco chloroplast genome - its gene organization and expression. EMBO J, 1986, 5: 2043-9. DOI:10.1002/j.1460-2075.1986.tb04464.x |
| [6] |
Mi H, Endo T, Schreiber U, et al. Donation of electrons from cytosolic components to the intersystem chain in the cyanobacterium Synechococcus sp. PCC 7002 as determined by the reduction of P700+. Plant Cell Physiol, 1992, 33: 1099-105. |
| [7] |
Mi HL, Endo T, Schreiber U, et al. Electron donation from cyclic and respiratory flows to the photosynthetic intersystem chain is mediated by pyridine nucleotide dehydrogenase in the cyanobacterium Synechocystis PCC 6803. Plant Cell Physiol, 1992, 33: 1233-7. |
| [8] |
Mi H, Endo T, Schreiber U, et al. NAD(P)H dehydrogenase- dependent cyclic electron flow around photosystem-Ⅰ in the cyanobacterium Synechocystis 6803 - a study of dark-starved cells and spheroplasts. Plant Cell Physiol, 1994, 35: 163-73. |
| [9] |
Mi H, Endo T, Ogawa T, et al. Thylakoid membrane-bound, NADPH-specific pyridine nucleotide dehydrogenase complex mediates cyclic electron transport in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol, 1995, 36: 661-8. |
| [10] |
Burrows PA, Sazanov LA, Svab Z, et al. Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. EMBO J, 1998, 17: 868-76. DOI:10.1093/emboj/17.4.868 |
| [11] |
Kofer W, Koop HU, Wanner G, et al. Mutagenesis of the genes encoding subunits A, C, H, I, J and K of the plastid NAD(P)H-plastoquinone-oxidoreductase in tobacco by polyethylene glycol-mediated plastome transformation. Mol Gen Genet, 1998, 258: 166-73. DOI:10.1007/s004380050719 |
| [12] |
Shikanai T, Endo T, Hashimoto T, et al. Directed disruption of the tobacco ndhB gene impairs cyclic electron flow around photosystem Ⅰ. Proc Natl Acad Sci U S A, 1998, 95: 9705-9. DOI:10.1073/pnas.95.16.9705 |
| [13] |
Horváth EM, Peter SO, Joet T, et al. Targeted inactivation of the plastid ndhB gene in tobacco results in an enhanced sensitivity of photosynthesis to moderate stomatal closure. Plant Physiol, 2000, 123: 1337-50. DOI:10.1104/pp.123.4.1337 |
| [14] |
Nixon PJ, Gounaris K, Coomber SA, et al. psbG is not a photosystem two gene but may be an ndh gene. J Biol Chem, 1989, 264: 14129-35. DOI:10.1016/S0021-9258(18)71652-9 |
| [15] |
Berger S, Ellersiek U, Westhoff P, et al. Studies on the expression of NDH-H, a subunit of the NAD(P)H- plastoquinone-oxidoreductase of higher-plant chloroplasts. Planta, 1993, 190: 25-31. |
| [16] |
Endo T, Mi HL, Shikanai T, et al. Donation of electrons to plastoquinone by NAD(P)H dehydrogenase and by ferredoxin-quinone reductase in spinach chloroplasts. Plant Cell Physiol, 1997, 38: 1272-7. DOI:10.1093/oxfordjournals.pcp.a029115 |
| [17] |
Munekage Y, Hojo M, Meurer J, et al. PGR5 is involved in cyclic electron flow around photosystem Ⅰ and is essential for photoprotection in Arabidopsis. Cell, 2002, 110: 361-71. DOI:10.1016/S0092-8674(02)00867-X |
| [18] |
Munekaga Y, Hashimoto M, Miyaka C, et al. Cyclic electron flow around photosystem Ⅰ is essential for photosynthesis. Nature, 2004, 429: 579-82. DOI:10.1038/nature02598 |
| [19] |
Shikanai T. Cyclic electron transport around photosystem Ⅰ: genetic approaches. Ann Rev Plant Biol, 2007, 58: 199-217. DOI:10.1146/annurev.arplant.58.091406.110525 |
| [20] |
Ogawa T, Mi H. Cyanobacterial NADPH dehydrogenase complexes. Photosynth Res, 2007, 93: 69-77. DOI:10.1007/s11120-006-9128-y |
| [21] |
Munekage Y, Hashimoto M, Miyake C, et al. Cyclic electron flow around photosystem Ⅰ is essential for photosynthesis. Nature, 2004, 429: 579-82. DOI:10.1038/nature02598 |
| [22] |
Dalcorso G, Pesaresi P, Masiero S, et al. A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell, 2008, 132: 273-85. DOI:10.1016/j.cell.2007.12.028 |
| [23] |
Hertle AP, Blunder T, Wunder T, et al. PGRL1 is the elusive ferredoxin-plastoquinone reductase in photosynthetic cyclic electron flow. Mol Cell, 2013, 49: 511-23. DOI:10.1016/j.molcel.2012.11.030 |
| [24] |
Schreiber UET, Mi HL, Asada K. Quenching analysis of chlorophyll fluorescence by the saturation pulse method: particular aspects relating to the study of eukayotic algae and cyanobacteria. Plant Cell Physiol, 1995, 36: 873-82. DOI:10.1093/oxfordjournals.pcp.a078833 |
| [25] |
Ma W, Ogawa T, Shen Y, et al. Changes in cyclic and respiratory electron transport by the movement of phycobilisomes in the cyanobacterium Synechocystis sp. strain PCC 6803. Biochim Biophys Acta, 2007, 1767: 742-9. DOI:10.1016/j.bbabio.2007.01.017 |
| [26] |
Nellaepalli S, Kodru S, Tirupathi M, et al. Anaerobiosis induced state transition: a non photochemical reduction of PQ pool mediated by NDH in Arabidopsis thaliana. PLoS One, 2012, 7: e49839. DOI:10.1371/journal.pone.0049839 |
| [27] |
Price GD, Badger MR, Woodger FJ, et al. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J Exp Bot, 2008, 59: 1441-61. DOI:10.1093/jxb/erm112 |
| [28] |
Mi H, Endo T, Schreiber U, et al. Electron donation from cyclic and respiratory flows to the photosynthetic intersystem chain is mediated by pyridine nucleotide dehydrogenase in the cyanobacterium Synechocystis PCC 6803. Plant Cell Physiol, 1992, 33: 1233-7. |
| [29] |
Han X, Sun N, Xu M, et al. Co-ordination of NDH and Cup proteins in CO2 uptake in cyanobacterium Synech-ocystis sp. PCC 6803. J Exp Botany, 2017, 68: 3869-77. DOI:10.1093/jxb/erx129 |
| [30] |
Deng Y, Ye JY, Mi HL. Effects of low CO2 on NAD(P)H dehydrogenase, a mediator of cyclic electron transport around photosystem Ⅰ in the cyanobacterium Synechocystis PCC6803. Plant Cell Physiol, 2003, 44: 534-40. DOI:10.1093/pcp/pcg067 |
| [31] |
Ma W, Deng Y, Ogawa T, et al. Active NDH-1 complexes from the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Cell Physiol, 2006, 47: 1432-6. |
| [32] |
Wang P, Duan W, Takabayashi A, et al. Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Physiol, 2006, 141: 465-74. |
| [33] |
Wang P, Ye J, Shen Y, et al. The role of chloroplast NAD(P)H dehydrogenase in protection of tobacco plant against heat stress. Sci China C Life Sci, 2006, 49: 311-21. |
| [34] |
Wu Y, He W, Ma W, et al. Low concentrations of NaHSO3 enhance NAD(P)H dehydrogenase-dependent cyclic photophosphorylation and alleviate the oxidative damage to improve photosynthesis in tobacco. Chin Sci Bull, 2012, 57: 3872-7. |
| [35] |
Wu Y, Zheng F, Ma W, et al. Regulation of NAD(P)H dehydrogenase-dependent cyclic electron transport around PSI by NaHSO3 at low concentrations in tobacco chloroplasts. Plant Cell Physiol, 2011, 52: 1734-43. |
| [36] |
Li N WJ, Li QH, Mi HL. Low concentration of NaHSO3 enhances the cyclic electron transport pathway mediated by PGR5/PGRL1 in Arabidopsis thaliana. Plant Physiol J, 2016, 52: 1745-51. |
| [37] |
Suorsa M, Jarvi S, Grieco M, et al. PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem Ⅰ to naturally and artificially fluctuating light conditions. Plant Cell, 2012, 24: 2934-48. |
| [38] |
Chen Q, Lan Y, Li Q, et al. Inactivation of photosynthetic cyclic electron transports upregulates photorespiration for compensation of efficient photosynthesis in Arabidopsis. Front Plant Sci, 2023, 14: 1061434. |
| [39] |
Wang F, Yan J, Ahammed GJ, et al. PGR5/PGRL1 and NDH mediate far-red light-induced photoprotection in response to chilling stress in tomato. Front Plant Sci, 2020, 11: 669. |
| [40] |
Chaturvedi AK, Dym O, Levin Y, et al. PGR5-LIKE PHOTOSYNTHETIC PHENOTYPE1A redox states alleviate photoinhibition during changes in light intensity. Plant Physiol, 2024, 194: 1059-74. |
| [41] |
Okegawa Y, Tsuda N, Sakamoto W, et al. Maintaining the chloroplast redox balance through the PGR5-dependent pathway and the Trx system is required for light-dependent activation of photosynthetic reactions. Plant Cell Physiol, 2022, 63: 92-103. |
| [42] |
Bradley RW, Bombelli P, Lea-Smith DJ, et al. Terminal oxidase mutants of the cyanobacterium Synechocystis sp. PCC 6803 show increased electrogenic activity in biological photo-voltaic systems. Phys Chem Chem Phys, 2013, 15: 13611-8. |
| [43] |
Gutekunst K, Chen X, Schreiber K, et al. The bidirectional NiFe-hydrogenase in Synechocystis sp. PCC 6803 is reduced by flavodoxin and ferredoxin and is essential under mixotrophic, nitrate-limiting conditions. J Biol Chem, 2014, 289: 1930-7. |
| [44] |
Appel J, Hueren V, Boehm M, et al. Cyanobacterial in vivo solar hydrogen production using a photosystem Ⅰ-hydrogenase (PsaD-HoxYH) fusion complex. Nat Energy, 2020, 5: 458-67. |
| [45] |
Yamamoto H, Takahashi S, Badger MR, et al. Artificial remodelling of alternative electron flow by flavodiiron proteins in Arabidopsis. Nat Plants, 2016, 2: 16012. |
| [46] |
Long TA, Okegawa Y, Shikanai T, et al. Conserved role of proton gradient regulation 5 in the regulation of PSI cyclic electron transport. Planta, 2008, 228: 907-18. |
| [47] |
Zhou L, Gao S, Wu S, et al. PGRL1 overexpression in Phaeodactylum tricornutum inhibits growth and reduces apparent PSⅡ activity. Plant J, 2020, 103: 1850-7. |
| [48] |
Dann M, Leister D. Evidence that cyanobacterial Sll1217 functions analogously to PGRL1 in enhancing PGR5-dependent cyclic electron flow. Nat Commun, 2019, 10: 5299. |
| [49] |
Wada S, Yamamoto H, Suzuki Y, et al. Flavodiiron protein substitutes for cyclic electron flow without competing CO2 assimilation in rice. Plant Physiol, 2018, 176: 1509-18. |
| [50] |
Kanai R, Edwards GE, Sage RF. The biochemistry of C4 photosynthesis[M]//Sage RF, Monson RK. C4 plant biology. San Diago: Academic Press, 1999: 49-87
|
| [51] |
Munekage YN, Eymery F, Rumeau D, et al. Elevated expression of PGR5 and NDH-H in bundle sheath chloroplasts in C4 flaveria species. Plant Cell Physiol, 2010, 51: 664-8. |
| [52] |
Zhao H, Wang Y, Lyv AM, et al. Two major metabolic factors for an efficient NADP-malic enzyme type C4 photosynthesis. Plant Physiol, 2022, 189: 84-98. |
| [53] |
Zhang Q, Tian S, Chen G, et al. Regulatory NADH dehydrogenase-like complex optimizes C4 photosynthetic carbon flow and cellular redox in maize. New Phytol, 2024, 241: 82-101. |
| [54] |
Tazoe Y, Ishikawa N, Shikanai T, et al. Overproduction of PGR5 enhances the electron sink downstream of photosystem Ⅰ in a C4 plant, Flaveria bidentis. Plant J, 2020, 103: 814-23. |
| [55] |
Seiml-Buchinger V, Reifschneider E, Bittner A. Ascorbate peroxidase postcold regulation of chloroplast NADPH dehydrogenase activity controls cold memory. Plant Physiol, 2022, 190: 1997-2016. |
| [56] |
Strand DD, Livingston AK, Satoh-Cruz M, et al. Activation of cyclic electron flow by hydrogen peroxide in vivo. Proc Natl Acad Sci U S A, 2015, 112: 5539-44. |
 2024, Vol. 36
2024, Vol. 36 

 米华玲,中国科学院分子植物科学卓越创新中心(前上海生命科学研究院植物生理生态研究所)植物分子遗传学国家重点实验室、光合与环境生物学实验室研究员,博士生导师,《植物生理与分子生物学学报》编委。长期以来从事类囊体膜NAD(P)H脱氢酶复合体(NDH-1)的结构与生理功能的研究,在国际上首次证明了蓝藻类囊体膜NDH-1介导围绕光系统Ⅰ的循环电子传递(Mi et al., 1992, 1994, 1995),两篇相关的论文入选1997年度日本植物生理学会论文奖;迄今,经常被国际学术刊物引用,2008年入选日本植物生理学界近50年影响度最高的论文。主持和参与多项国家基金项目,包括国家自然科学基金委面上项目、科技部重点研发项目、中国科学院先导B项目、农业部转基因专项等。研究组以蓝藻、水稻和拟南芥为模式系统,在分子水平、蛋白质水平和生化水平上研究关键调控蛋白的作用机理。主要研究方向包括:类囊体膜NADPH脱氢酶复合体的结构与功能研究、CO2浓缩机制的调控机理研究
米华玲,中国科学院分子植物科学卓越创新中心(前上海生命科学研究院植物生理生态研究所)植物分子遗传学国家重点实验室、光合与环境生物学实验室研究员,博士生导师,《植物生理与分子生物学学报》编委。长期以来从事类囊体膜NAD(P)H脱氢酶复合体(NDH-1)的结构与生理功能的研究,在国际上首次证明了蓝藻类囊体膜NDH-1介导围绕光系统Ⅰ的循环电子传递(Mi et al., 1992, 1994, 1995),两篇相关的论文入选1997年度日本植物生理学会论文奖;迄今,经常被国际学术刊物引用,2008年入选日本植物生理学界近50年影响度最高的论文。主持和参与多项国家基金项目,包括国家自然科学基金委面上项目、科技部重点研发项目、中国科学院先导B项目、农业部转基因专项等。研究组以蓝藻、水稻和拟南芥为模式系统,在分子水平、蛋白质水平和生化水平上研究关键调控蛋白的作用机理。主要研究方向包括:类囊体膜NADPH脱氢酶复合体的结构与功能研究、CO2浓缩机制的调控机理研究